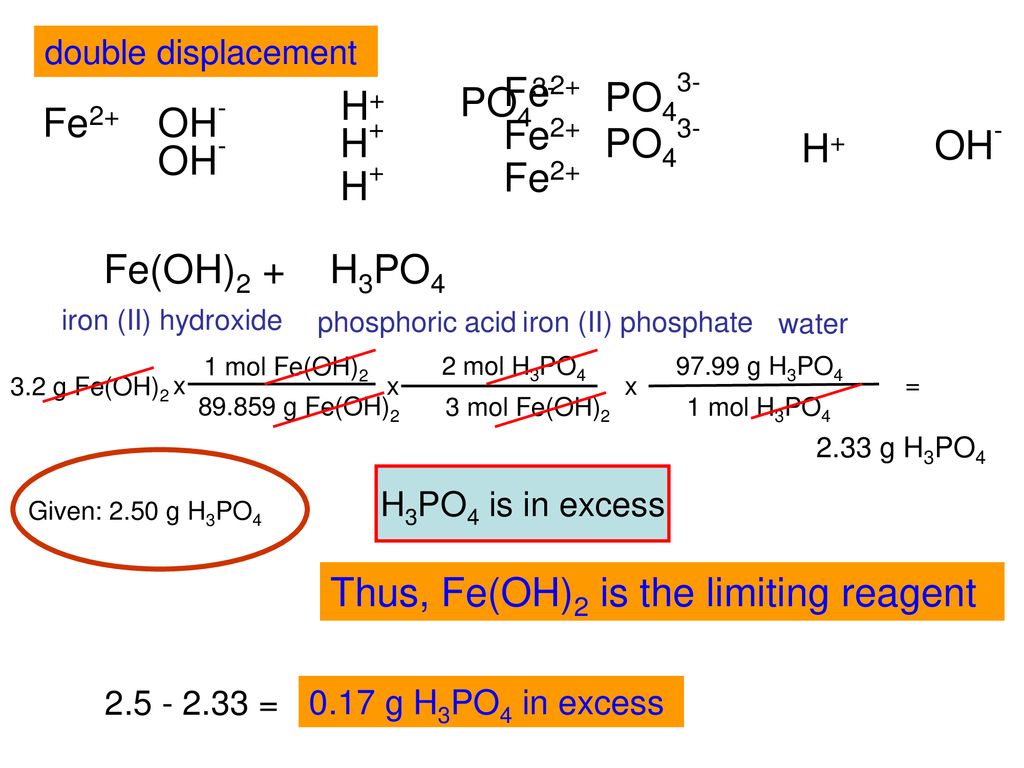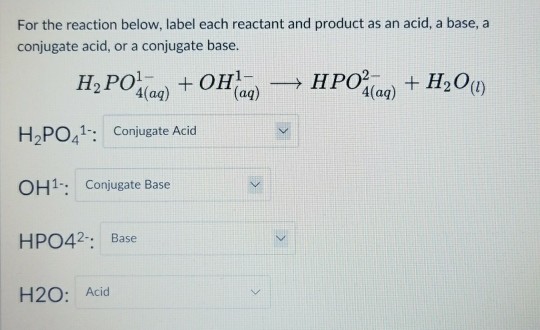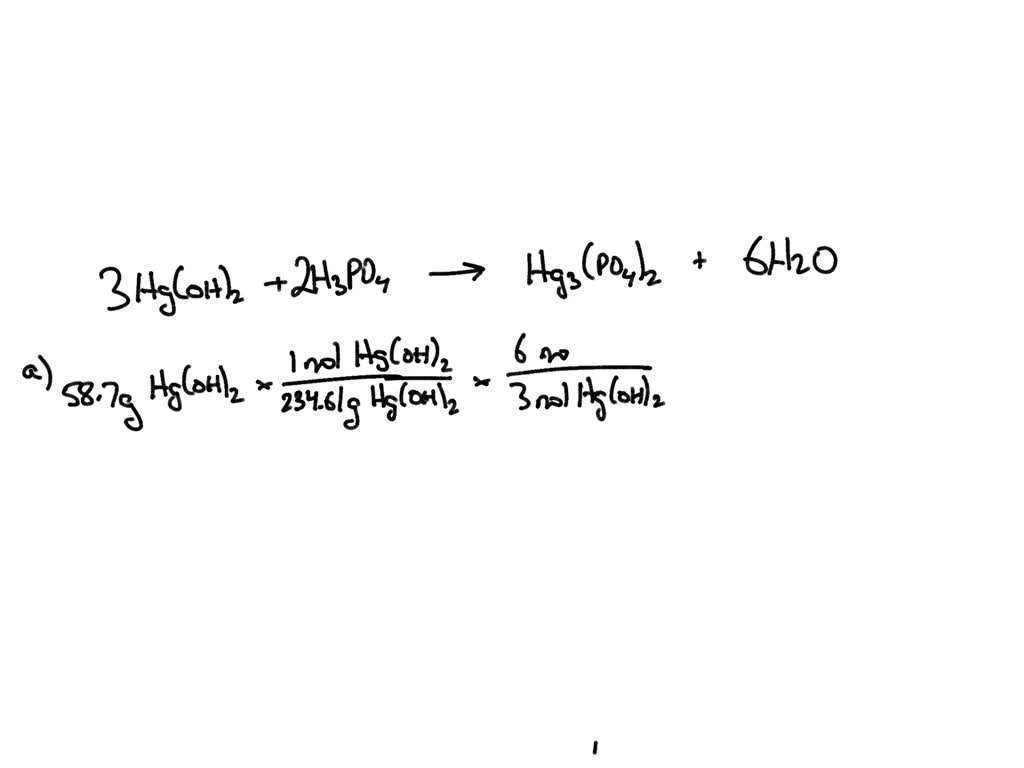
SOLVED: Use the reaction to solve the following problems. Hg(OH)2 + H3PO4→ Hg3(PO4)2 + H2O A) Given 58.7 grams of Hg(OH)2, how many moles of H2O will be produced? If you react
Magnetic susceptibility and crystal structure of the new oxovanadium phosphate (NH4)Zn(H2O)(VO)2(PO4)2(H2PO4)

Synthesis and Characterization of a Novel Hydrated Layered Vanadium(III) Phosphate Phase K3V3(PO4)4·H2O: A Functional Cathode Material for Potassium-Ion Batteries | ACS Omega
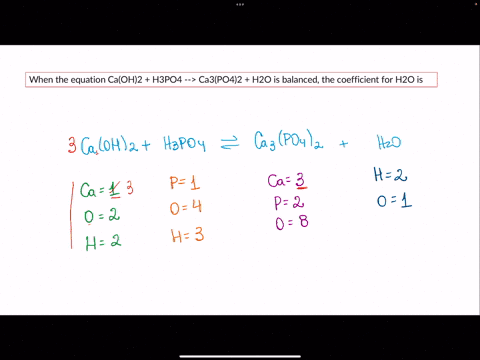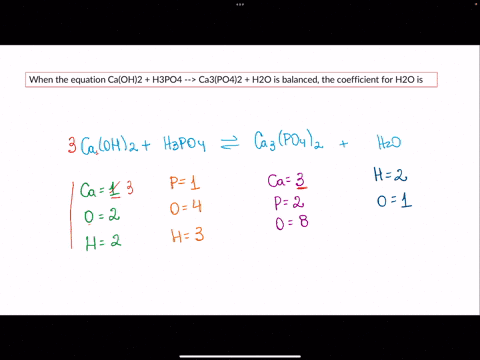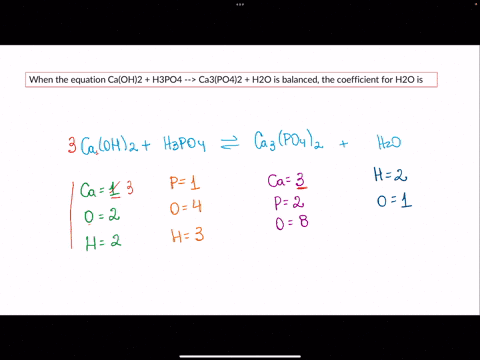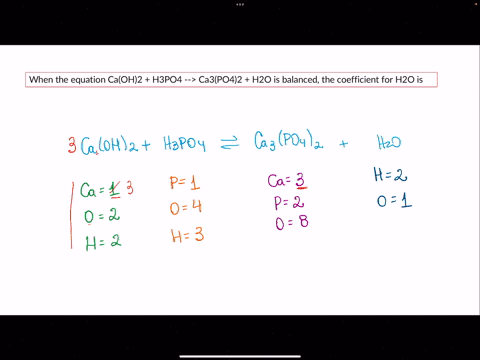
SOLVED: When the equation Ca(OH)2 + H3PO4 –> Ca3(PO4)2 + H2O is balanced, the coefficient for H2O is
![Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer | Inorganic Chemistry Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/acs.inorgchem.1c02685/asset/images/acs.inorgchem.1c02685.social.jpeg_v03)
Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer | Inorganic Chemistry

The experimental X-Ray diffraction pattern of Fe3(PO4)2.8H2O, allowed... | Download Scientific Diagram

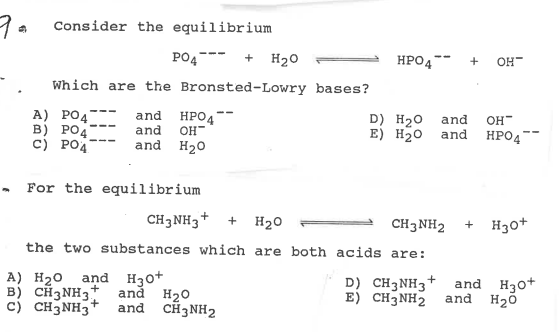
42. Three reactions involving H,PO, are given below 1. H,PO4 + H20 - H30* + H2PO4 II. H POE +H20- HPO2 + H20+ III. HỌPO4 + OH - H3PO4 +02- In which

Reproducibility of five identical Ni3(PO4)2.8H2O thin films electrode... | Download Scientific Diagram

Spex CertiPrep AS-PO49-2Y Phosphate (PO4)-3 Single-Element Ion Anion Standard, 1,000 µg/mL in H2O; 125 mL from Cole-Parmer India





![Crandallite(Ca[Al3(OH)5(PO4)2].H2O) 1318-36-1 wiki Crandallite(Ca[Al3(OH)5(PO4)2].H2O) 1318-36-1 wiki](https://structimg.guidechem.com/1/1/290580.png)