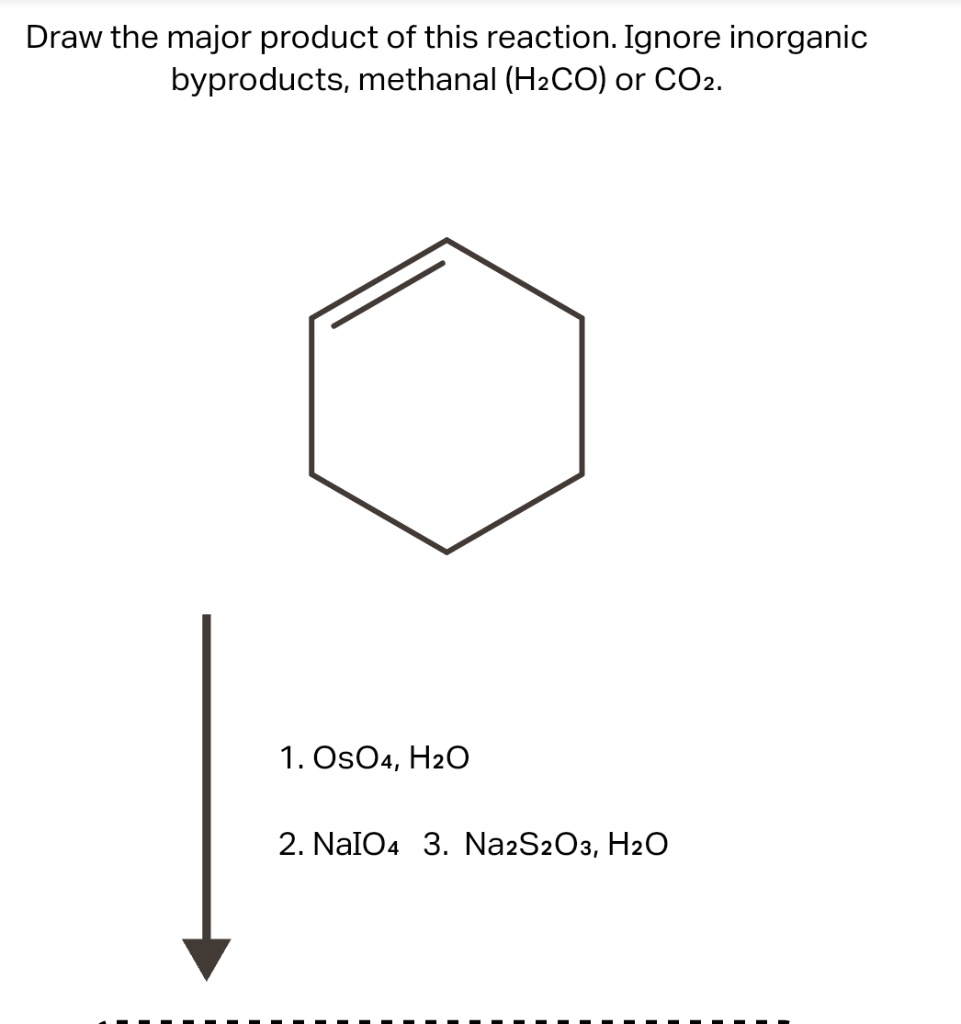
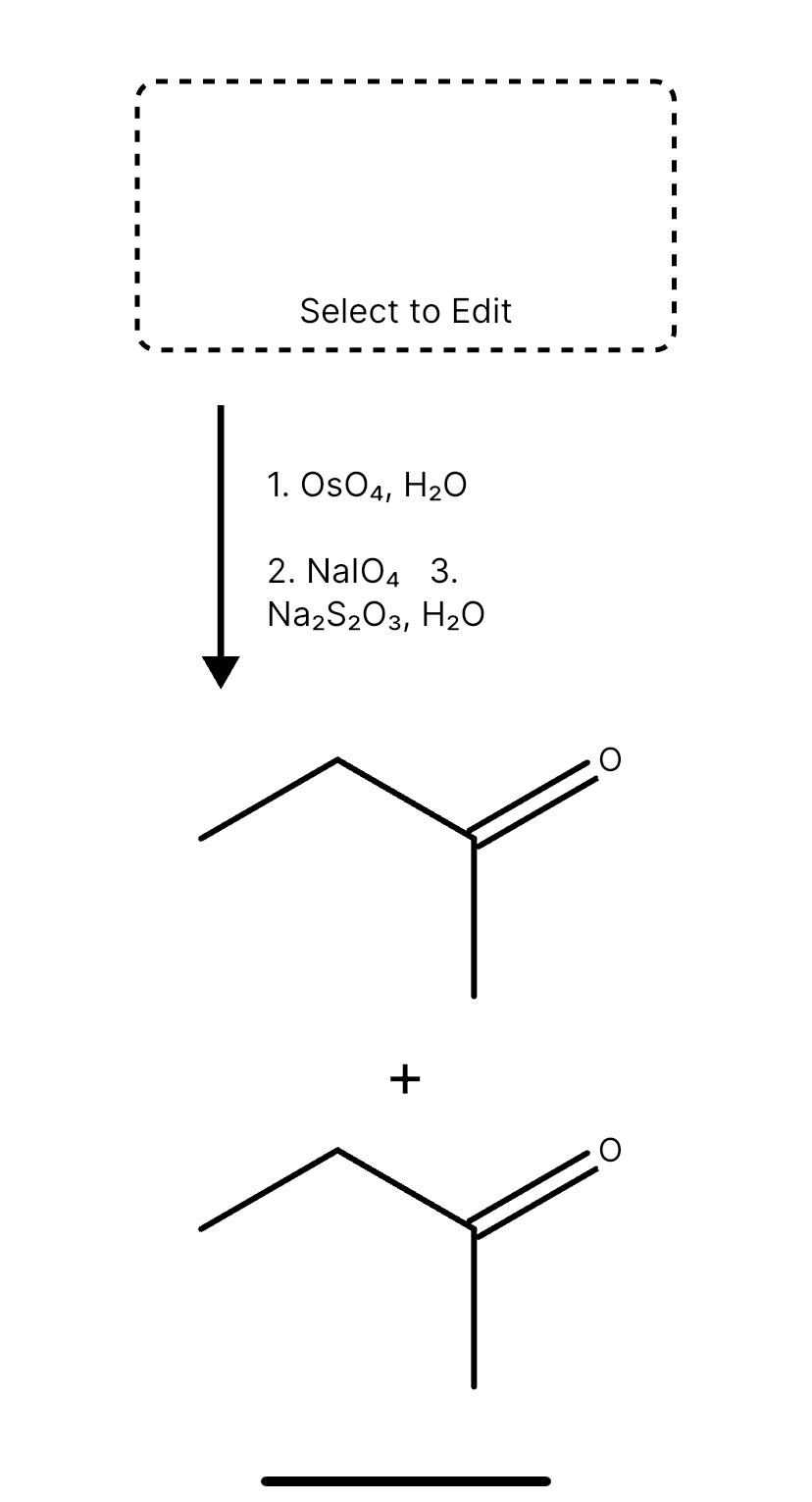
Question No. 88 Na2S2O3 has the molar mass M. In the reaction Na2S2O3 + H2O + Cl2 → Na2SO4 + 2HCI + S the equivalent weight of Na2S203 is :- Marks: +4 / -1

View In Na2S2O3 has the molar mass M. In the reaction Na2S2O3 + H2O + Cl2 → Na2SO4 + 2HCl + S the equivalent weight of Na2S2O3 is :- (1)M

Cyclic voltammogram of a 0.3 M CdCl2·H2O + 0.03 M Na2S2O3·5H2O and b... | Download Scientific Diagram

help why is the exchange of the number of electrons for na2s2o3 in this reaction 8 and not 2 1uhli5ii -Chemistry - TopperLearning.com

333. The system Na2S2O3–Ag2S2O3–H2O at 25° - Journal of the Chemical Society (Resumed) (RSC Publishing)

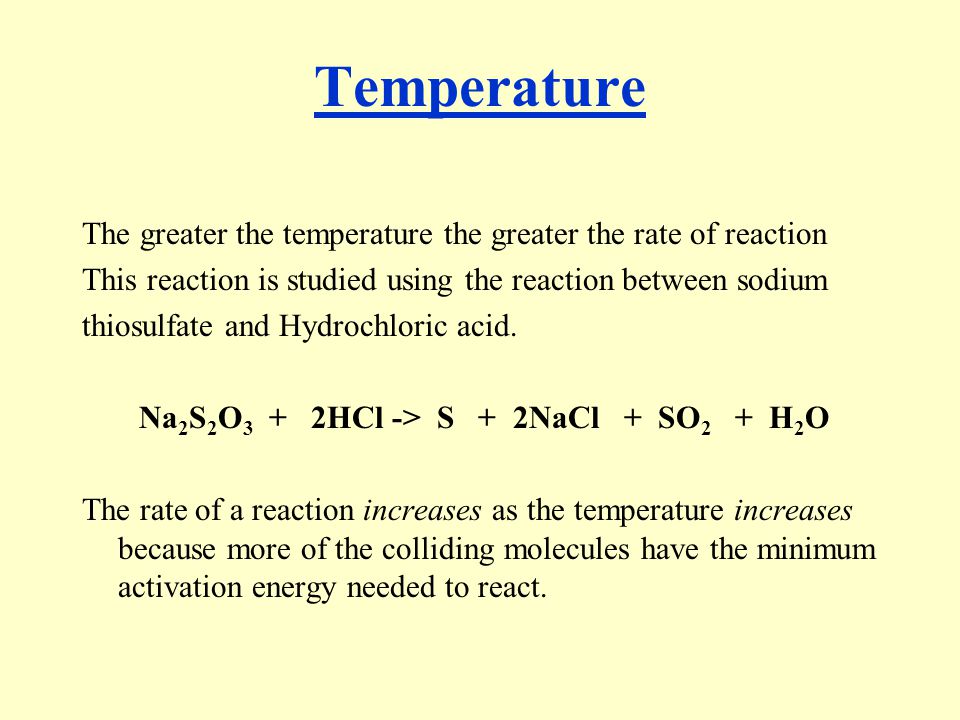
The Rate of Reaction Between Sodium Thiosulphate and Hydrochloric Acid | PDF | Reaction Rate | Chemical Reactions
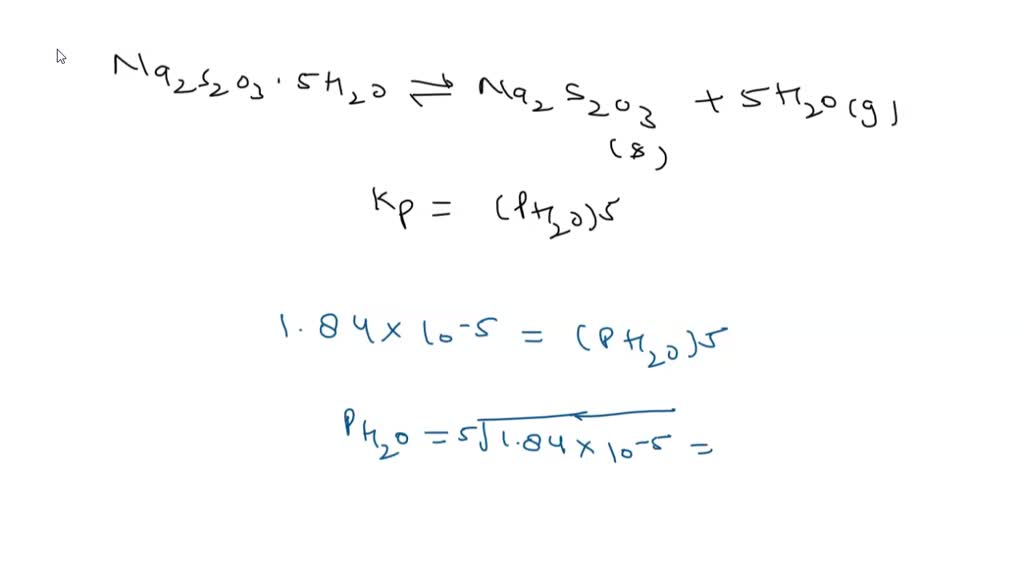
SOLVED: Sodium thiosulfate pentahydrate (FM 248.18 g ∙ mol-1) loses water when it is heated in an oven: Na2S2O3∙5H2O(s) ⇌ Na2S2O3(s) + 5H2O(g). ΔH° and ΔS° for this reaction at 25°C are

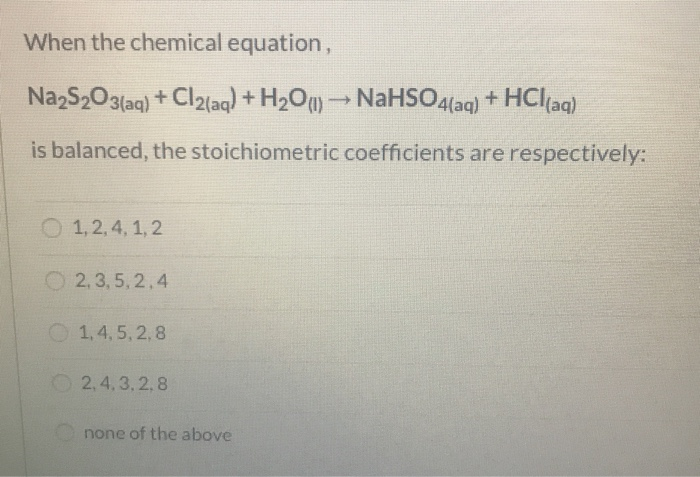
Aqueous solution of Na2S2O3 on reaction with Cl2 gives: NO 2 S 03 +12 (1) Na2S406 (2) NaHSO4 (3) NaCl (4) NaOH