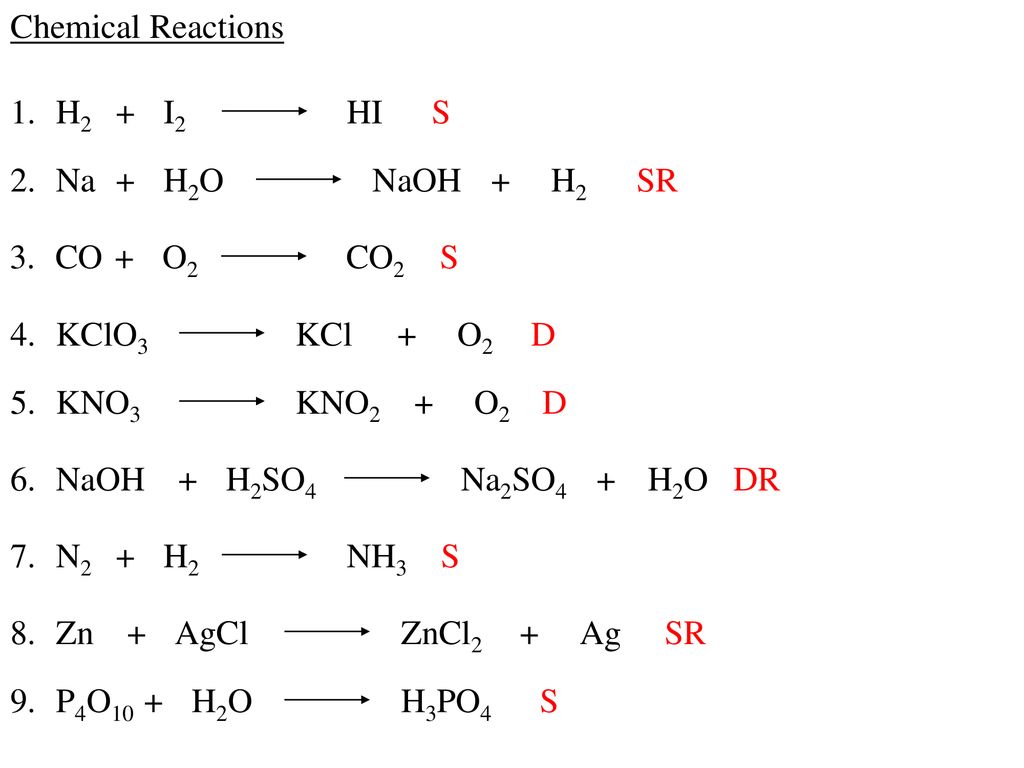
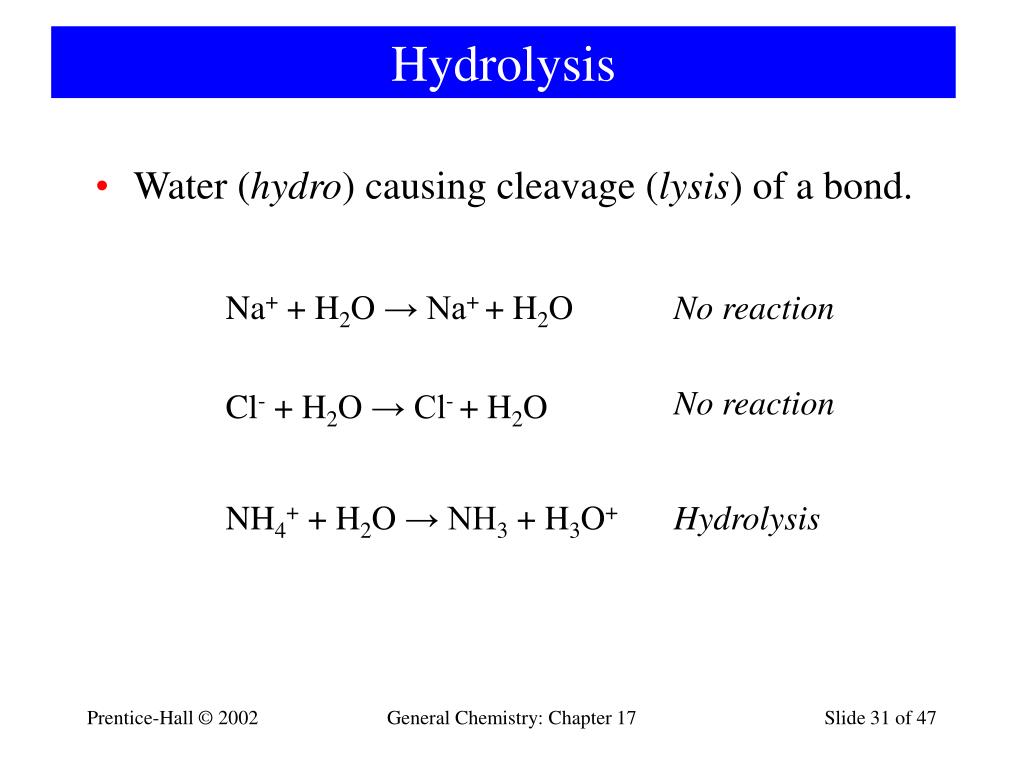
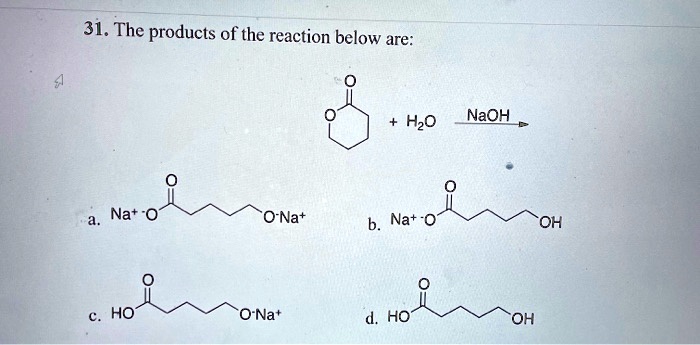
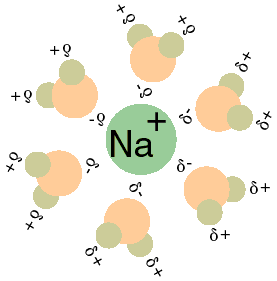Study of the solubility, viscosity and density in Na+, Zn2+/Cl− − H2O, Na+ − Zn2+ − (H2PO2)− − H2O, Na+, Cl−/(H2PO2)− − H2O, and Zn2+, Cl−/(H2PO2)− − H2O ternary systems, and in
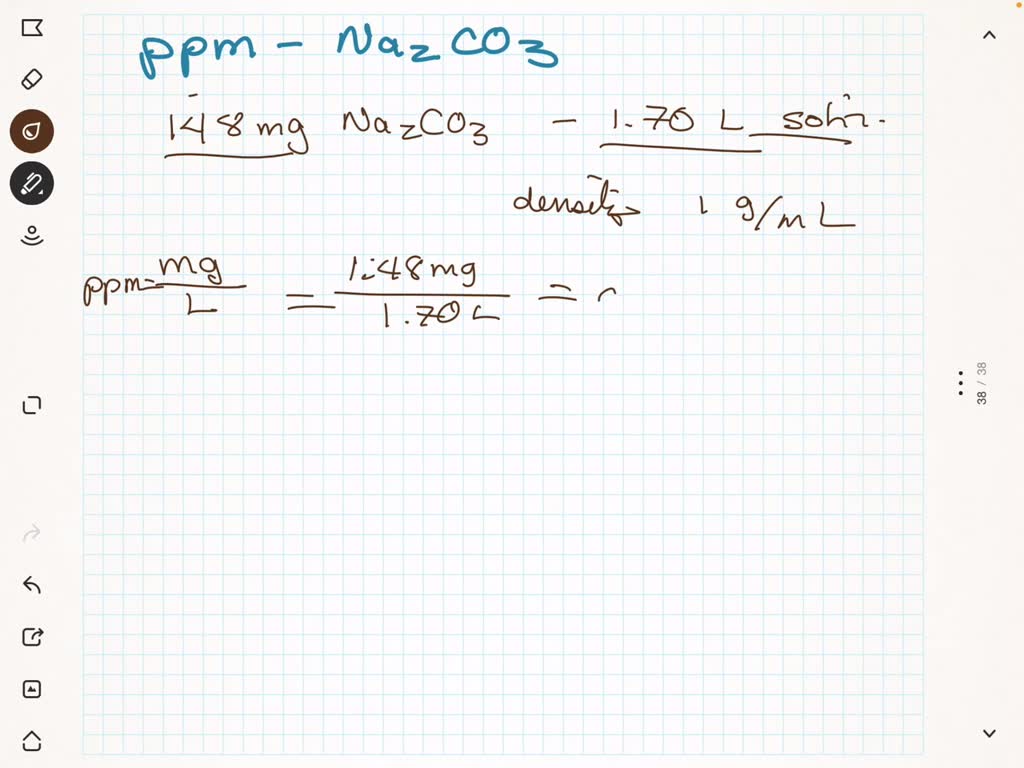
SOLVED: A 182-mg sample of Na2CO3 is dissolved in H2O to give 2.75L of solution. What is the concentration of Na+ in parts per million (ppm)? Note: "ppm" are 106 ×[(mass of
![na+ h2o = Noah +h2. [tex]na + h2o - - - - \: naoh \: + h2 \: \\ balanced \: it \: class10[/tex] - Brainly.in na+ h2o = Noah +h2. [tex]na + h2o - - - - \: naoh \: + h2 \: \\ balanced \: it \: class10[/tex] - Brainly.in](https://hi-static.z-dn.net/files/d25/926242c076a095778521da06b382ea61.jpg)
na+ h2o = Noah +h2. [tex]na + h2o - - - - \: naoh \: + h2 \: \\ balanced \: it \: class10[/tex] - Brainly.in
Sodium standard pour Chromatographie d'ion, TraceCERT®, 1000 mg/L Na+ dans H2O, SUPELCO® - Materiel pour Laboratoire

Structures of (a) H2O, (b) Mg(H2O)62+, and (c) Fe(H2O)63+ and (d) the... | Download Scientific Diagram
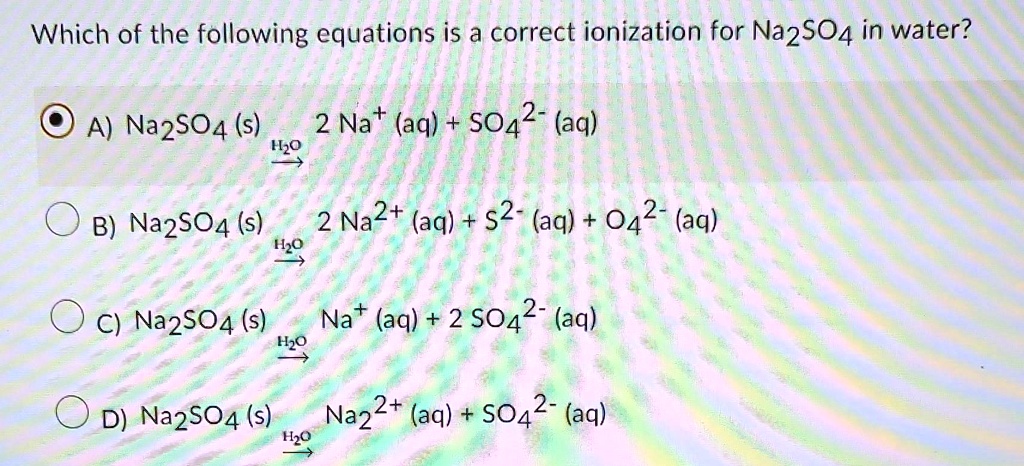
SOLVED: please help thanks a lot Which of the following equations is a correct ionization for Na2SO4 in water? A) Na2SO4(s) 2Na+aq+SO42-aq H2O B) Na2SO4(s) 2 Na2+(aq)+s2-(aq)+O42-(aq) H20 ) C) Na2SO4(s) Na+(aq)+2
![Frontiers | Hydrated Sodium Ion Clusters [Na+(H2O)n (n = 1–6)]: An ab initio Study on Structures and Non-covalent Interaction Frontiers | Hydrated Sodium Ion Clusters [Na+(H2O)n (n = 1–6)]: An ab initio Study on Structures and Non-covalent Interaction](https://www.frontiersin.org/files/Articles/468925/fchem-07-00624-HTML/image_m/fchem-07-00624-t001.jpg)