Proposed transformation pathways of BPA in the Mn2O3/PMS system. First,... | Download Scientific Diagram

How many grams Mn2O3 would be produced from the complete reaction of 46.8 g of MnO2 ? Zn + 2MnO2 + H2O - brainly.com
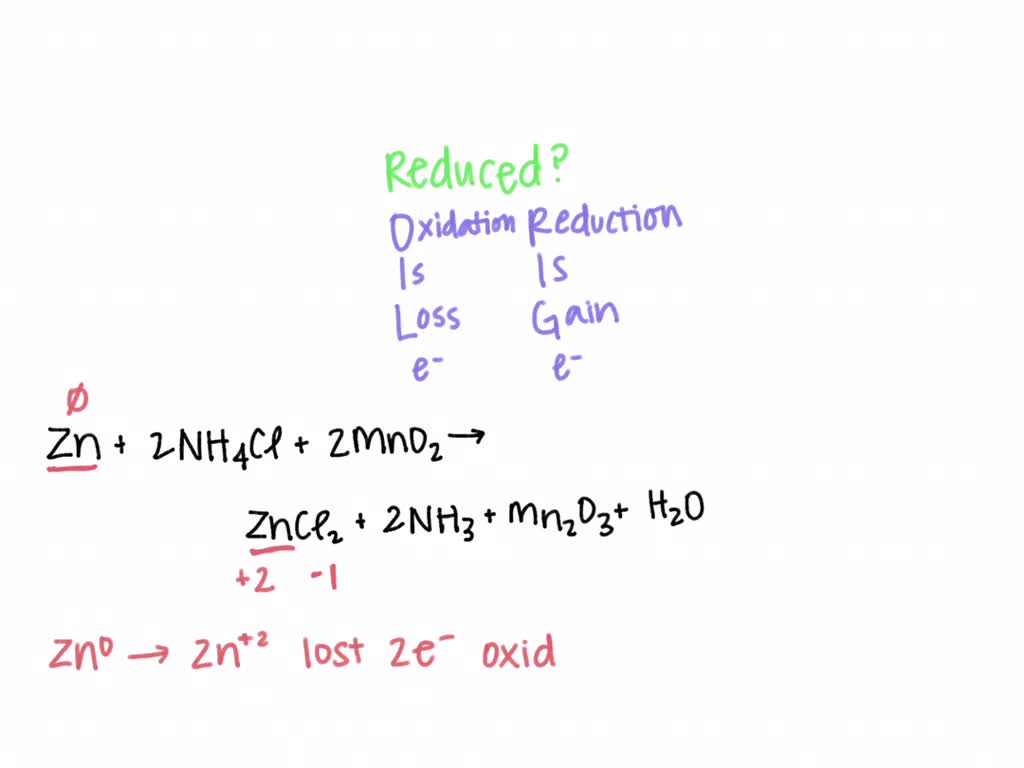
SOLVED: In an acid dry cell battery for which the reaction is Zn + 2 NH4Cl + 2 MnO2 ? ZnCl2 + 2 NH3 + Mn2O3 + H2O, what atom is reduced?
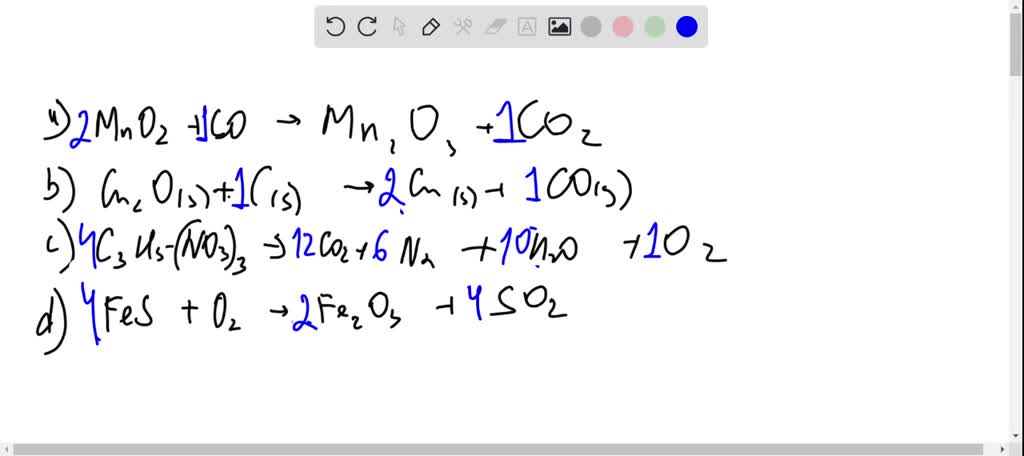
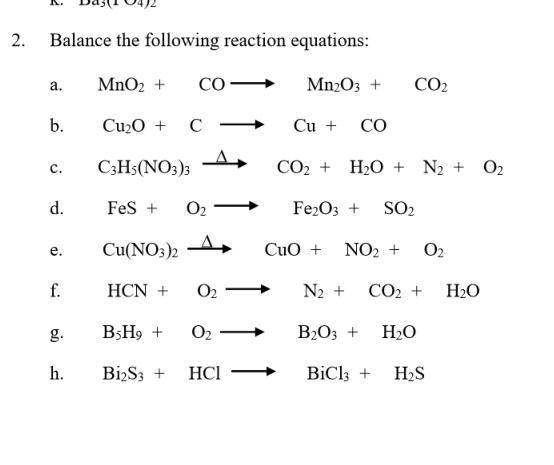
SOLVED: Balance the following equations: (a) MnO2 + CO â†' Mn2O3 + CO2 (b) Cu2O(s) + C(s) â†' Cu(s) + CO(g) (c) C3H5(NO3)3 â†' CO2 + H2O + N2 + O2 (d)

Evaluation of MnOx, Mn2O3, and Mn3O4 Electrodeposited Films for the Oxygen Evolution Reaction of Water | The Journal of Physical Chemistry C

Understanding hydrothermal transformation from Mn2O3 particles to Na0.55Mn2O4·1.5H2O nanosheets, nanobelts and single crystalline ultra-long Na4Mn9O18 nanowires | Scientific Reports
Density functional theory calculated reaction pathways of (a) Mn2O3 +... | Download Scientific Diagram

Understanding hydrothermal transformation from Mn2O3 particles to Na0.55Mn2O4·1.5H2O nanosheets, nanobelts and single crystalline ultra-long Na4Mn9O18 nanowires | Scientific Reports

Calcium Manganese(III) Oxides (CaMn2O4⋅x H2O) as Biomimetic Oxygen‐Evolving Catalysts - Najafpour - 2010 - Angewandte Chemie International Edition - Wiley Online Library
How many grams Mn2O3 would be produced from the complete reaction of 46.8 g of MnC 2 ? Zn+ [algebra]
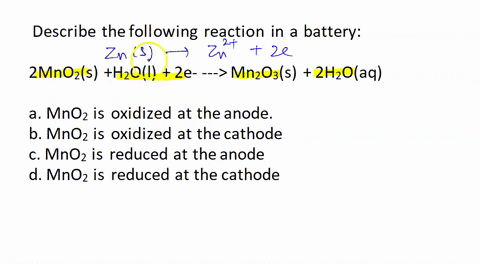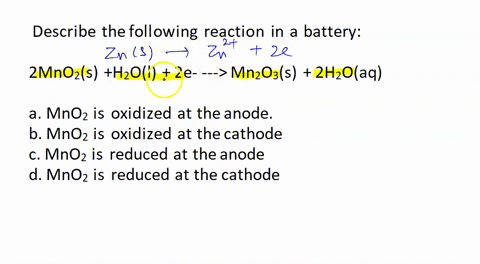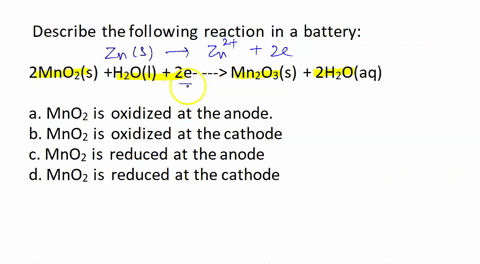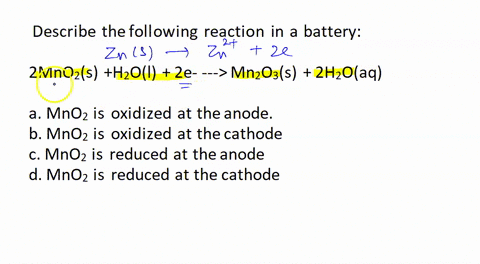
SOLVED: Describe the following reaction in a battery: 2 MnO2(s) + H2O(l) + 2e- —> Mn2O3(s) + 2 H2O(aq) a. MnO2 is oxidized at the anode. b. MnO2 is reduced at the








![How many grams Mn2O3 would be produced from the complete reaction of 46.8 g of M || 102 [algebra] How many grams Mn2O3 would be produced from the complete reaction of 46.8 g of M || 102 [algebra]](https://p16-ehi-va.gauthmath.com/tos-maliva-i-ejcjvp0zxf-us/33c8cdf452324a458870f75e4227d3cb~tplv-ejcjvp0zxf-webp.webp)



![Solved [3] Manganese dry cell Cathode 2MnO2 + 2H+ + 2e → | Chegg.com Solved [3] Manganese dry cell Cathode 2MnO2 + 2H+ + 2e → | Chegg.com](https://media.cheggcdn.com/study/a0a/a0a0ef03-1105-49d9-a45f-969fa87d8d7f/image)