
calculate the equilibrium constant of H2 + O2 gives us H2O + CEO at 13957 if the equilibrium constant 135 - Chemistry - Equilibrium - 13886927 | Meritnation.com

Question⬇️ Does “moles of hydrogen gas” refer to moles of H2? Meaning that 2H2 is 2 moles of hydrogen gas? : r/chemhelp

Consider the following reaction at certain temperature: H2O(g)+CO2(g) equilibrium to H2(g)+CO2(g) Some molecules of H2O and CO are placed in a 1.0 L container as shown below. When equilibrium is reached, how

Direct production of H2O2 from H2 and O2 in a biphasic H2O/scCO2 system over a Pd/C catalyst: Optimization of reaction conditions - ScienceDirect

31) The equilibrium constant the reaction H2O + CO) = H2(g) + CO2(g) is 0.44 1260K. The equilibrium constant the reaction 2H2(g) + 2C029) 7=2C0g + 2H2O(g) 1260 K is equal to

Mechanistic studies of methanol synthesis over Cu from CO/CO2/H2/H2O mixtures: The source of C in methanol and the role of water - ScienceDirect

85. For the reaction, 2NO + 2H2 — N2 + 2H20, the mechanism is given below 2NO=N2O2 N2O2 + H2 - slow > N2O + H2O 4 ) N2O + H2 -
![SOLVED: Determine the equilibrium-constant expression for the reaction: CuO(s) + H2(g) ⇌ Cu(l) + H2O(l) ? A. K = [H2O]/[CuO] B. K = [Cu][H2O]/[H2][CuO] C. K = 1/[H2] D. K = [H2]/[Cu] SOLVED: Determine the equilibrium-constant expression for the reaction: CuO(s) + H2(g) ⇌ Cu(l) + H2O(l) ? A. K = [H2O]/[CuO] B. K = [Cu][H2O]/[H2][CuO] C. K = 1/[H2] D. K = [H2]/[Cu]](https://cdn.numerade.com/ask_previews/69a6a069-64fe-4b52-b6aa-a926786b3f81_large.jpg)
SOLVED: Determine the equilibrium-constant expression for the reaction: CuO(s) + H2(g) ⇌ Cu(l) + H2O(l) ? A. K = [H2O]/[CuO] B. K = [Cu][H2O]/[H2][CuO] C. K = 1/[H2] D. K = [H2]/[Cu]



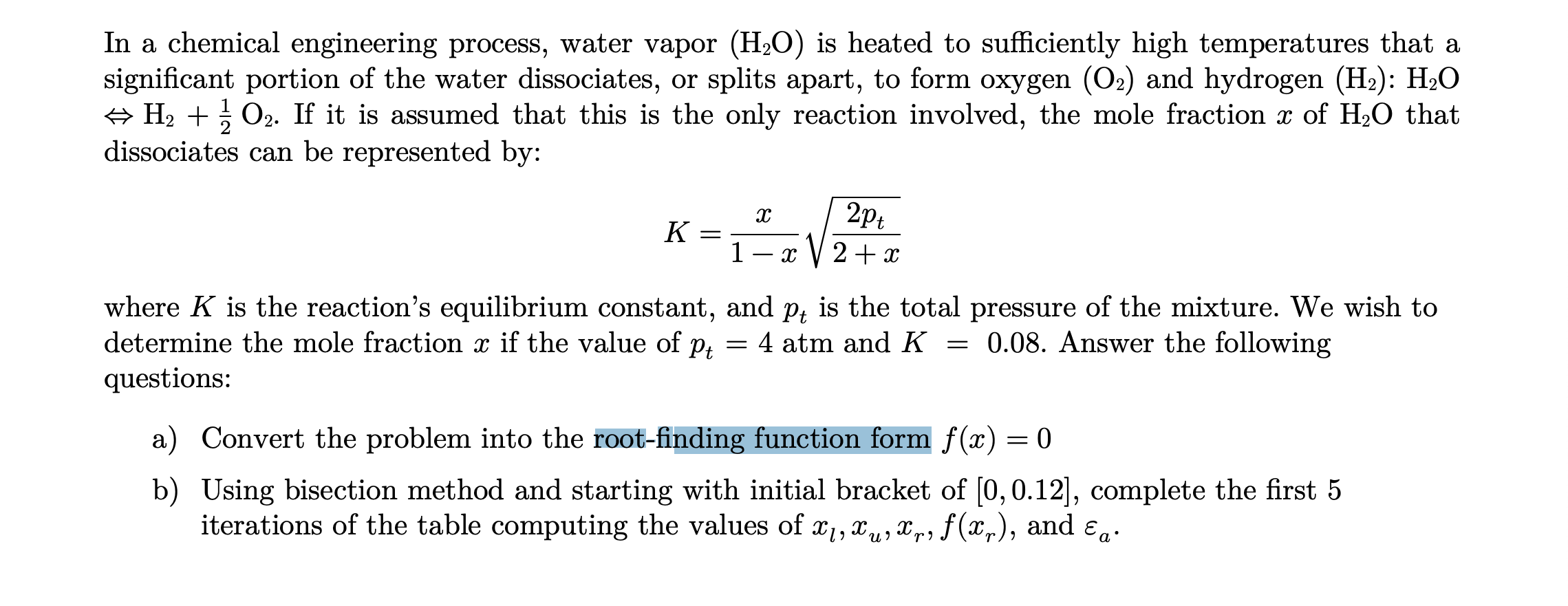



![PDF] Two triple points in the H2O–H2 system† | Semantic Scholar PDF] Two triple points in the H2O–H2 system† | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0e3a3f978107aae0e7b1ab47beca2a2ce22bd231/3-Figure2-1.png)

