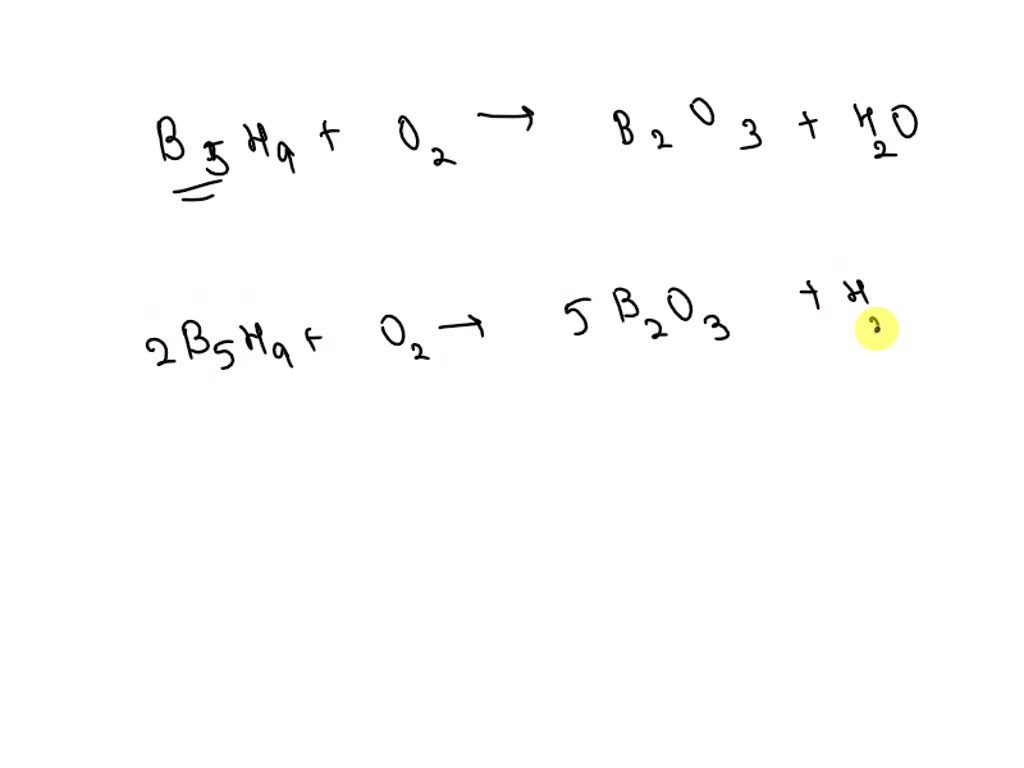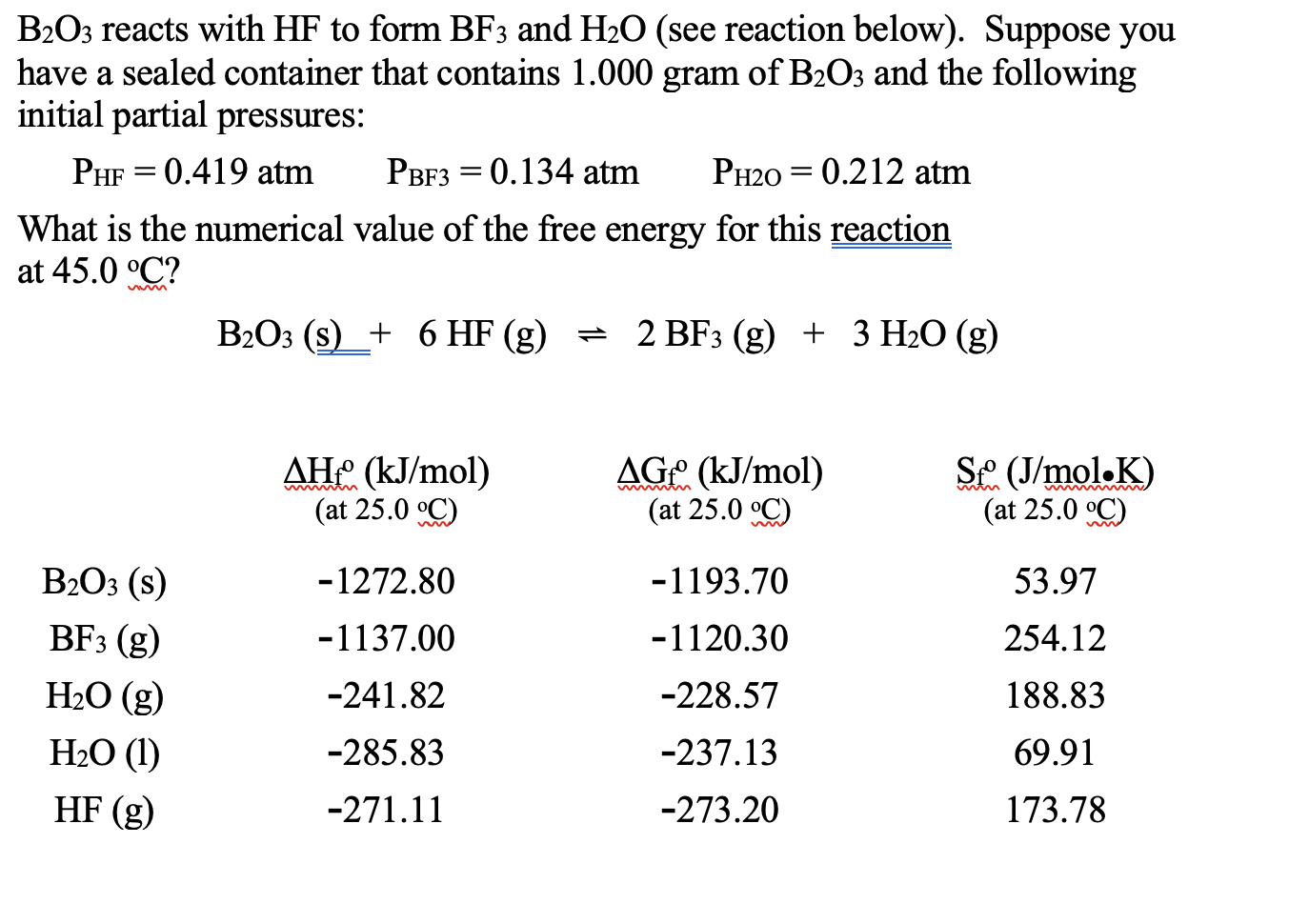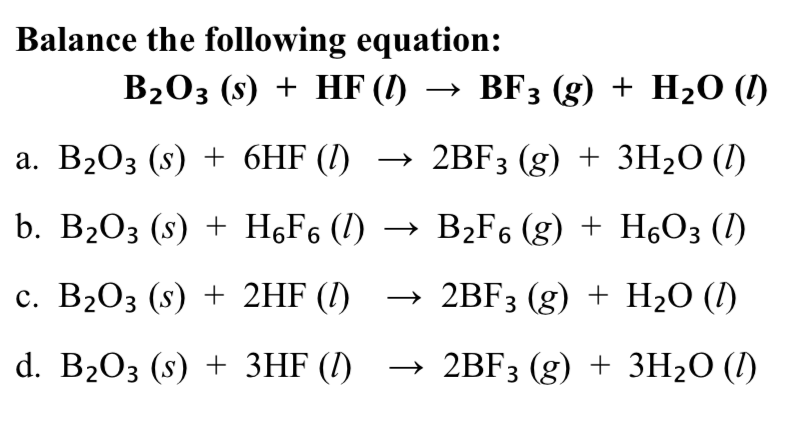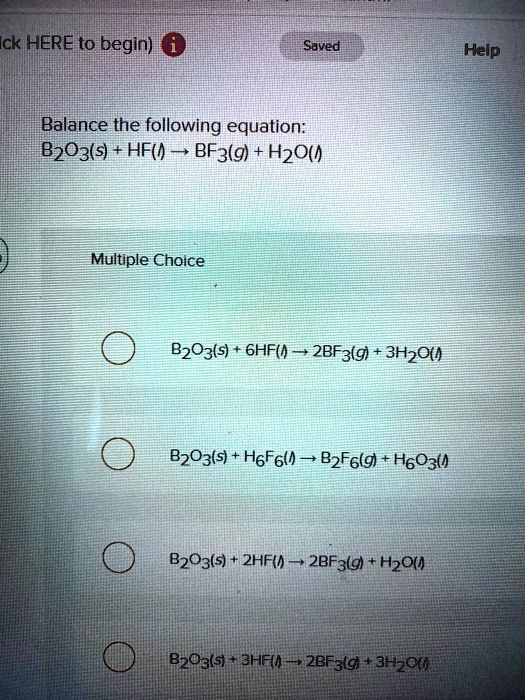
SOLVED: Click HERE to begin. Saved Help Balance the following equation: B2O3(s) + HF(g) â†' BF3(g) + H2O(l) Multiple Choice B2O3(s) + 6HF(g) â†' 2BF3(g) + 3H2O(l) B2O3(s) + 6HF(g) â†' B2F6(g) +
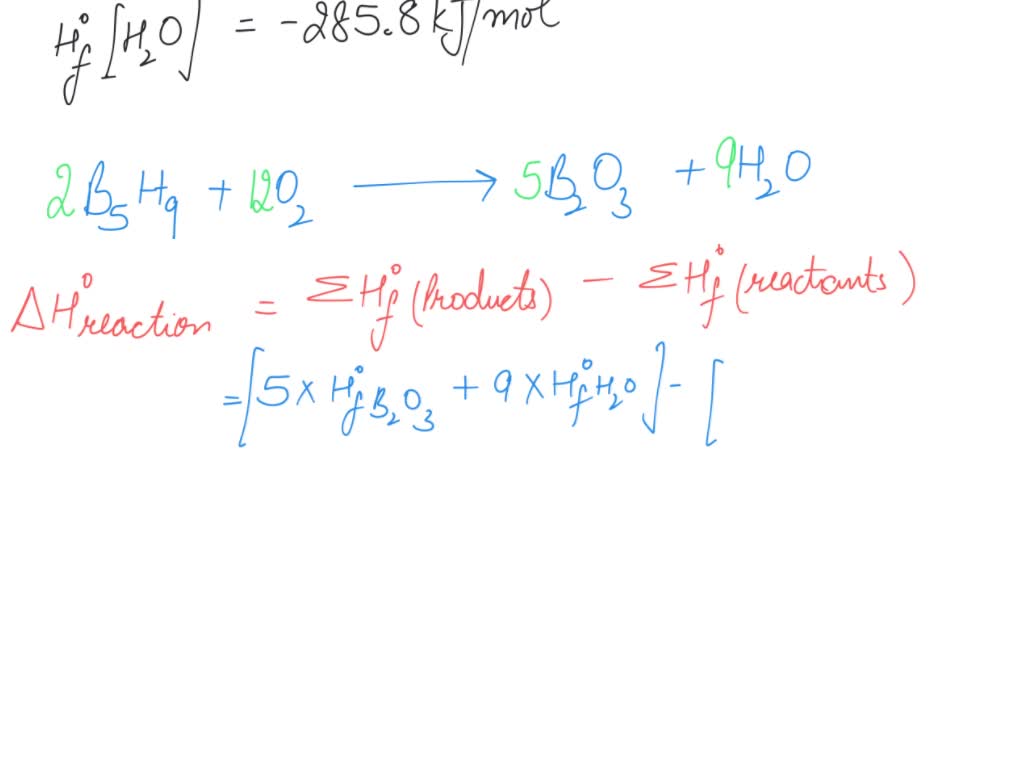
SOLVED: Pentaborane B5H9(s) burns vigorously in O2 to give B2O3(s) and H2O(l). What is ΔH° for the combustion of 1 mol of B5H9(s)? Substance ΔH°f (kJ/mol) B2O3(s) –1273.5 B5H9(s) +73.2 H2O(l) –285.8

B2O3 + 3 H2O ---> 2 H3BO3 If diboron trioxide is reacted with water, the product is boric acid. What mass of boric acid is obtained from the full reaction. - ppt video online download

Novel 4ZnO⋅B2O3⋅H2O:Ln3+/HTC (where Ln = Eu or Tb) phosphors: Synthesis, morphology and luminescence properties | Journal of Materials Research | Cambridge Core

B2O3 + 3 H2O ---> 2 H3BO3 If diboron trioxide is reacted with water, the product is boric acid. What mass of boric acid is obtained from the full reaction. - ppt video online download

Rate constants determination for reactions of B2O3 with H2O and HCl at high temperature using ab initio calculations - ScienceDirect

B2H6 O2 (A) + H2O 35. H2OHBO3 + (B) o- On Select the correct option: (1) (A) = B2O3, (B) = HBO2 (2) (A) = B2O3, (B) = O2 (3) (A) = B, (B) = H2 (4) (A) = B203, (B) = H2

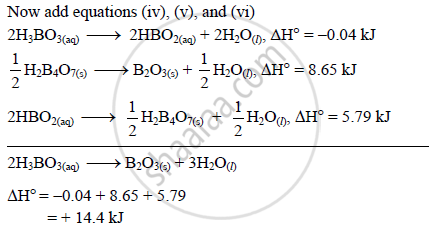
H3B03 on heating decomposes in two ways: 1. HBO3 + HBO2 + H2O II. H2B03 → B2O3 + H2O If 9 moles of H3BO3 are taken, some part decomposed like (1) and
![8-Diagramme d'équilibre de phase B2O3-SiO2 d'après Rockett et al. [RF65] | Download Scientific Diagram 8-Diagramme d'équilibre de phase B2O3-SiO2 d'après Rockett et al. [RF65] | Download Scientific Diagram](https://www.researchgate.net/publication/326029008/figure/fig12/AS:642325411807240@1530153720175/Diagramme-dequilibre-de-phase-B2O3-SiO2-dapres-Rockett-et-al-RF65.png)
8-Diagramme d'équilibre de phase B2O3-SiO2 d'après Rockett et al. [RF65] | Download Scientific Diagram