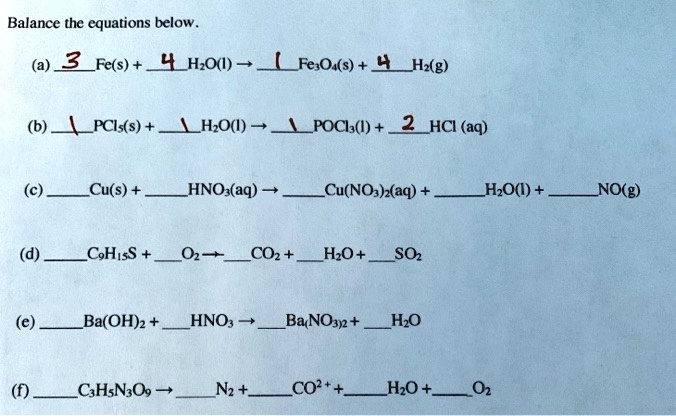
Al + KMnO4 + H2SO4 = KHSO4 + Al2(SO4)3 + MnSO4 + H2O KNO3 + FeSO4 + H2SO4 = KHSO4 + Fe2(SO4)3 + NO H2O H2S + K2Cr2O7 + H2SO4 =KHSO4 + Cr2(SO4)3 + S + H2O Balance the equations using ion electron method.
Eau , H2O, molécule HOH.Il s'agit d'un composé hydroxy inorganique, un hydrure d'oxygène composé d'un atome d'oxygène et de deux atomes d'hydrogène.Produit chimique structural f Image Vectorielle Stock - Alamy

Complete the following chemical reactions.(i) PbS(s)+H2O2(aq)→(ii) MnO−4(aq)+H2O2(aq)→(iii) CaO(s)+H2O(g)→(Iv) AlCl3(g)+H2O(l)→(v) Ca3N2(s)+H2O(l)→Classify the above into (a) hydrolysis, (b) redox and (c) hydration reaction.
in which reaction delS is positive 1.H2O(l)=H2O(s) 2.3O2(g)=2O3(g) 3.H2O(l)= H2O(g) 4.N2 (g)+3H2(g)=2NH3(g)
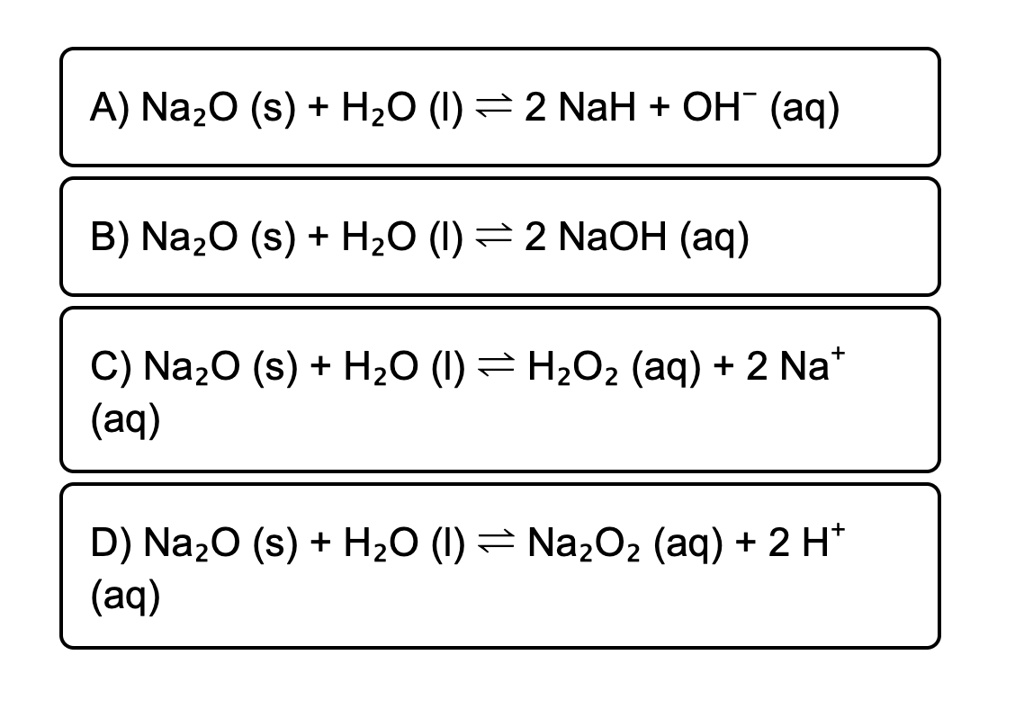
SOLVED: A) Na2O (s) + H2O (l) = 2 NaOH (aq) B) Na2O (s) + H2O (l) = 2 NaOH (aq) C) Na2O (s) + H2O (l) = H2O2 (aq) + 2
![surr Entropy changes the process, H,09 → H2O(s) normal pressure and 274 K are given below AS sustem = -22.13, ASgurr = +22.05, the process is non-spontaneous because [AMU (Med.) 2009] (a) surr Entropy changes the process, H,09 → H2O(s) normal pressure and 274 K are given below AS sustem = -22.13, ASgurr = +22.05, the process is non-spontaneous because [AMU (Med.) 2009] (a)](https://toppr-doubts-media.s3.amazonaws.com/images/7995954/386c6b10-083b-4da3-8077-07544ef23d55.jpg)
surr Entropy changes the process, H,09 → H2O(s) normal pressure and 274 K are given below AS sustem = -22.13, ASgurr = +22.05, the process is non-spontaneous because [AMU (Med.) 2009] (a)

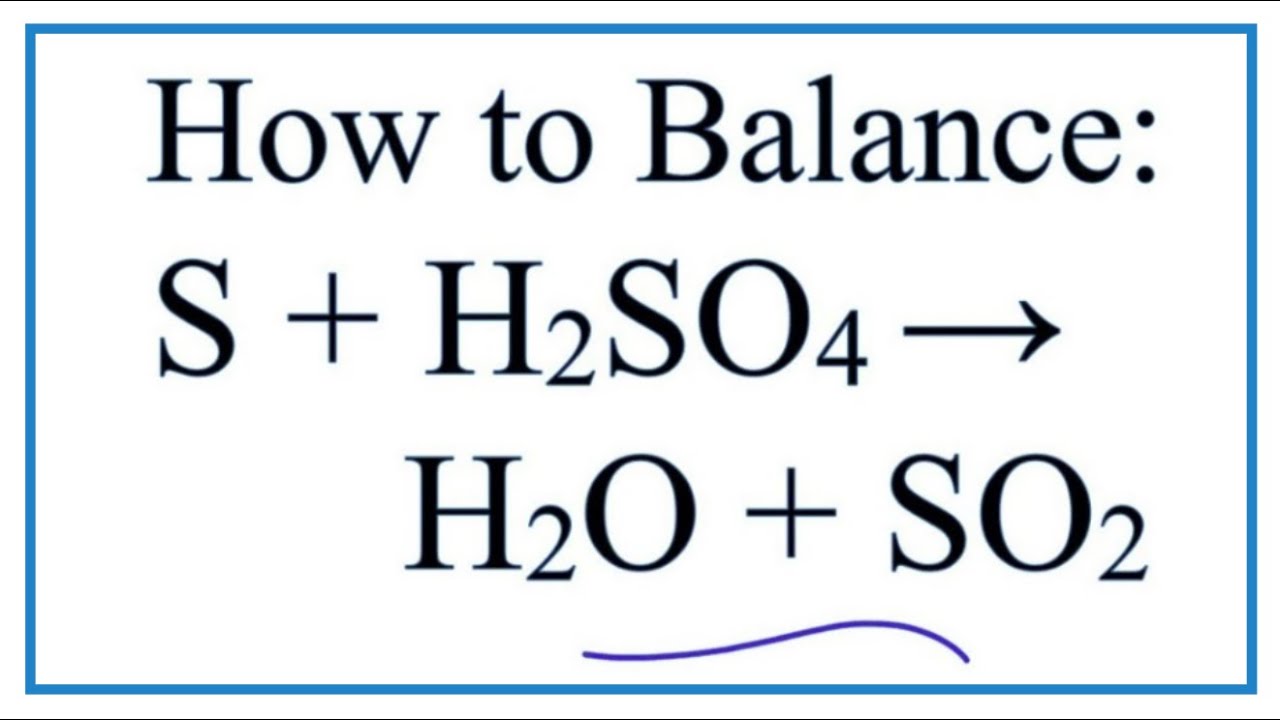
H2S+SO2=H2O+S Balanced Equation||Hydrogen sulphide+Sulphur dioxide=Water+Sulphur Balanced Equation - YouTube

H2O Audio Écouteurs étanches pour la Natation - Surge S+ (câble Court) écouteurs Piscine Noir/Bleu : Amazon.fr: High-Tech

Molécule D'eau H2o Hoh. Il S'agit D'un Hydroxy-composé Inorganique D'oxygène Hydrure Composé D'un Atome D'oxygène Et De Deux Illustration de Vecteur - Illustration du moléculaire, structurel: 232952086

Equilibrium reactions and thermodynamic constant of the Sc-S-H2O system... | Download Scientific Diagram
Eau , H2O, molécule HOH.Il s'agit d'un composé hydroxy inorganique, un hydrure d'oxygène composé d'un atome d'oxygène et de deux atomes d'hydrogène.Produit chimique structural f Image Vectorielle Stock - Alamy
H2S + KMnO4 + H2SO4 = S + MnSO4 + K2SO4 + H2O Can anyone balance it by oxidation number method along with proper explanation? - Quora