FDA Drug Safety Communication: Addition of another concentration of liquid acetaminophen marketed for infants | FDA
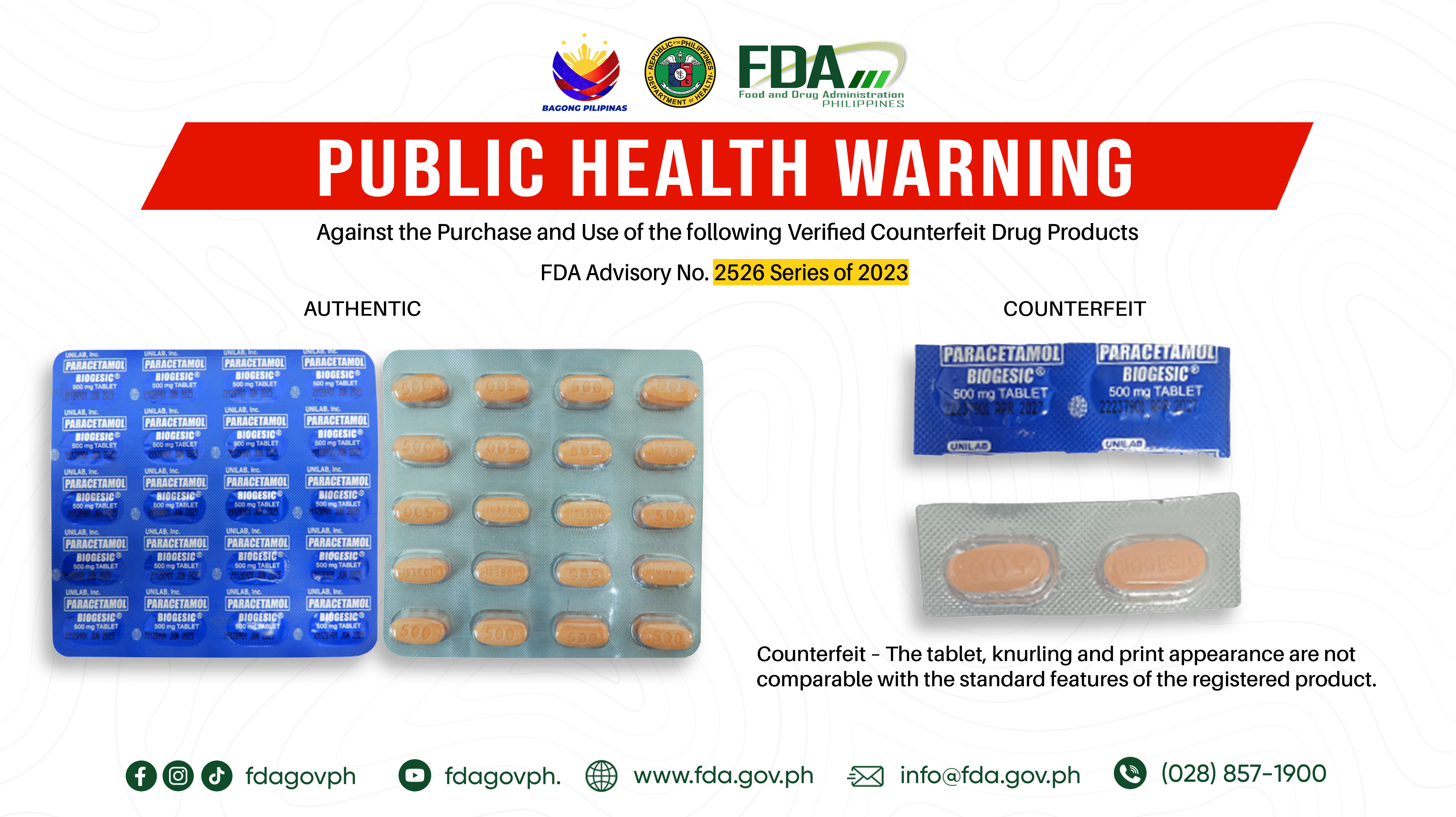
FDA Advisory No.2023-2526 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2023-1931 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

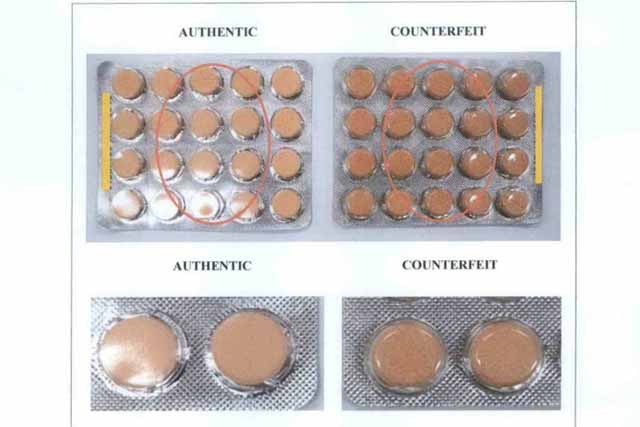
FDA Advisory No.2022-0135 || Public Health Warning Against the Purchase and Use of the Counterfeit Drug Product “Paracetamol (Biogesic®) 500 mg Tablet” - Food and Drug Administration

Les comprimés de paracétamol BPF La FDA a approuvé l'allégement 350mg - Chine Le paracétamol, l'Acétaminophène

FDA Advisory No.2023-1838 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2022-0008 || Public Health Warning Against the Purchase and Use of the Verified Counterfeit Drug Product “Phenylpropanolamine HCl/ Chlorphenamine Maleate/ Paracetamol (Decolgen® Forte) 25 mg/ 2 mg/ 500 mg Tablet” -

FDA Advisory No.2023-2327 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

Le paracétamol Comprimés 500 mg / 325mg BPF La FDA a approuvé le paracétamol antipyrétique - Chine Le paracétamol, l'Acétaminophène

FDA Advisory No.2022-0134 || Public Health Warning Against the Purchase and Use of the Verified Counterfeit Drug Product “Phenylephrine HCl/ Chlorphenamine Maleate/ Paracetamol (Bioflu®) 10 mg/ 2 mg/ 500 mg Film-Coated Tablet” -
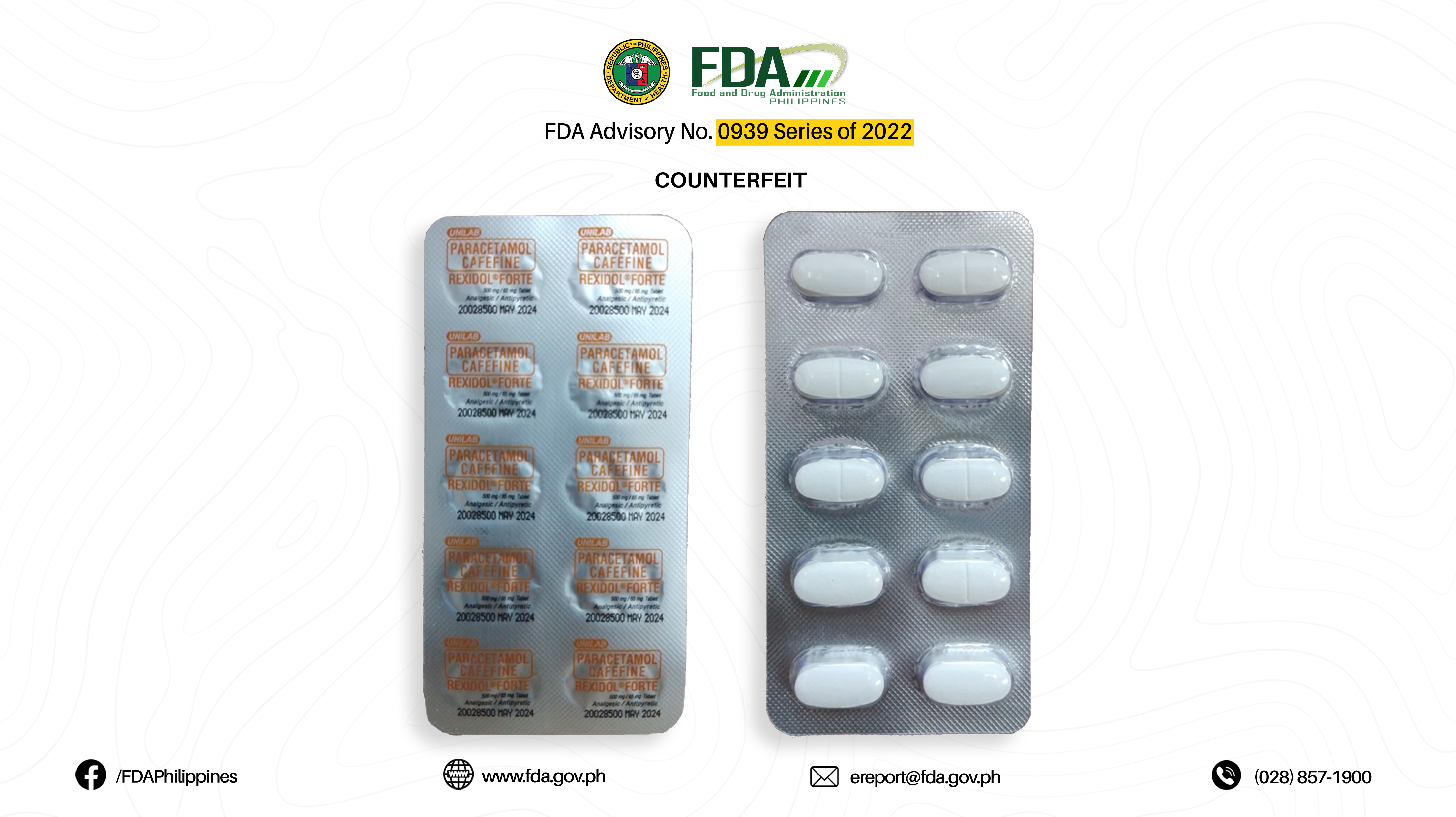
FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

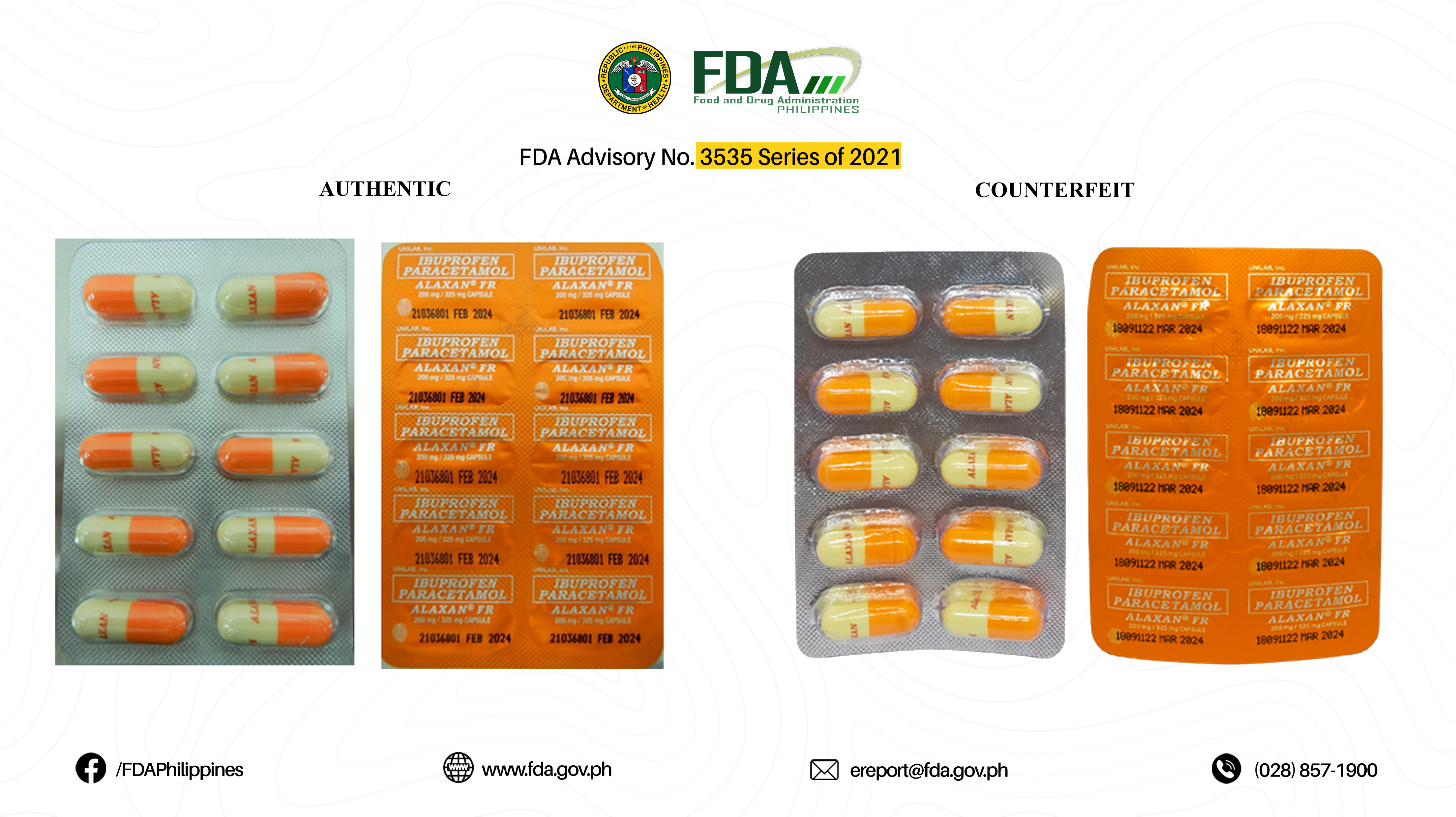
FDA Advisory No.2022-0620 || Public Health Warning Against the Purchase and Use of the Counterfeit Drug Product “Ibuprofen/ Paracetamol (Alaxan® FR) 200 mg / 325 mg Capsule” - Food and Drug Administration

FDA Advisory No.2023-1840 || Public Health Warning on Substandard (Contaminated) Paracetamol + Phenylephrine Chlorhydrate + Chlorpheniramine Maleate Syrup Confirmed by the World Health Organization (WHO) - Food and Drug Administration

FDA Advisory No.2022-0779 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration