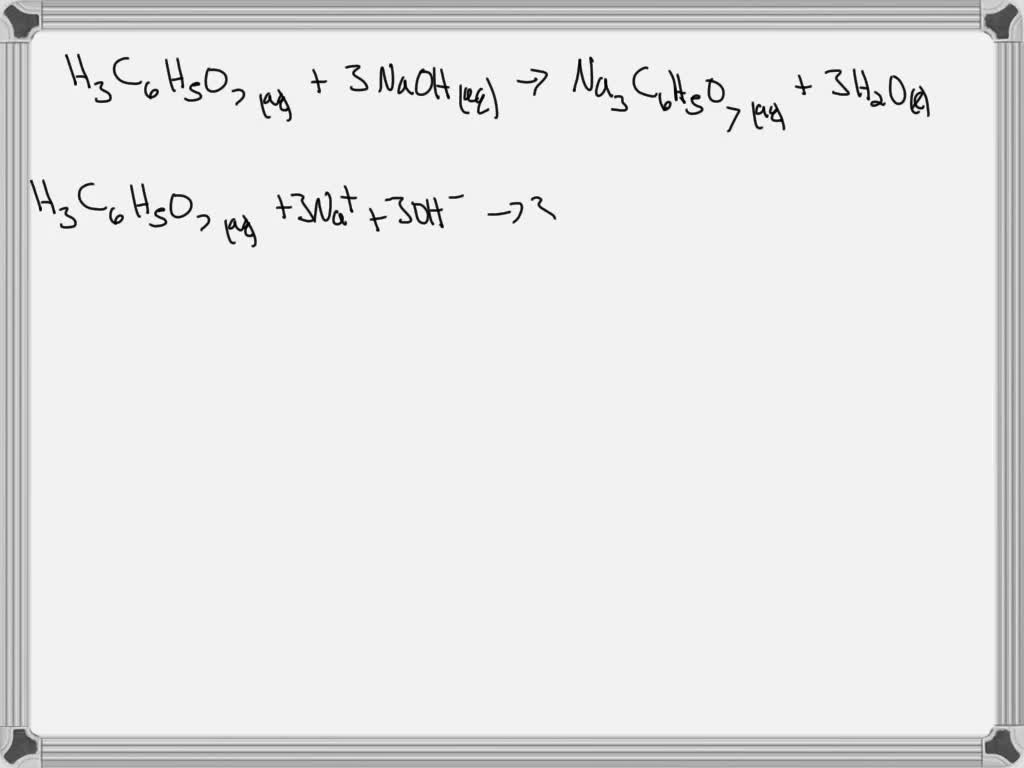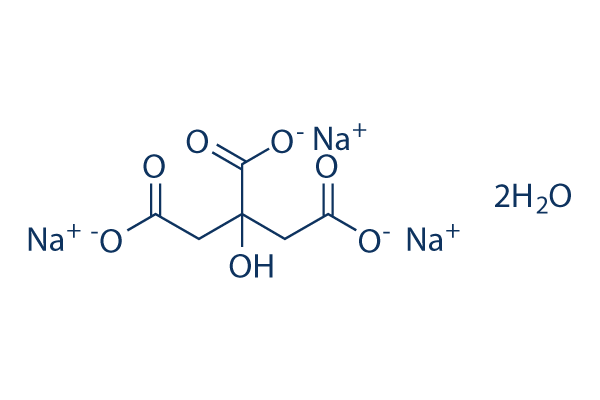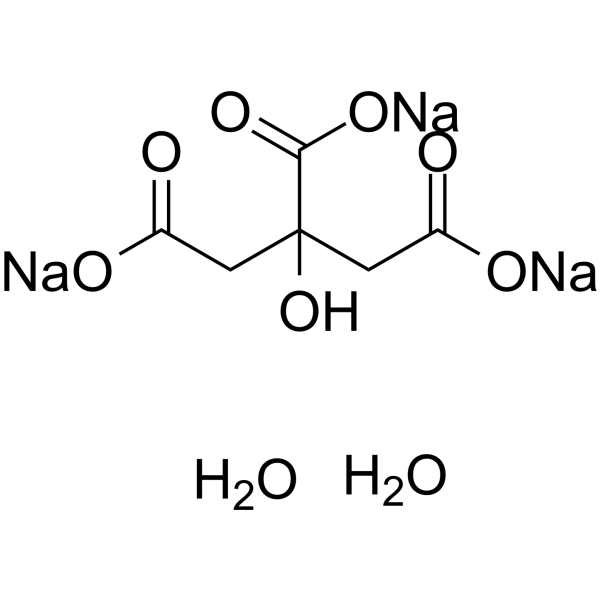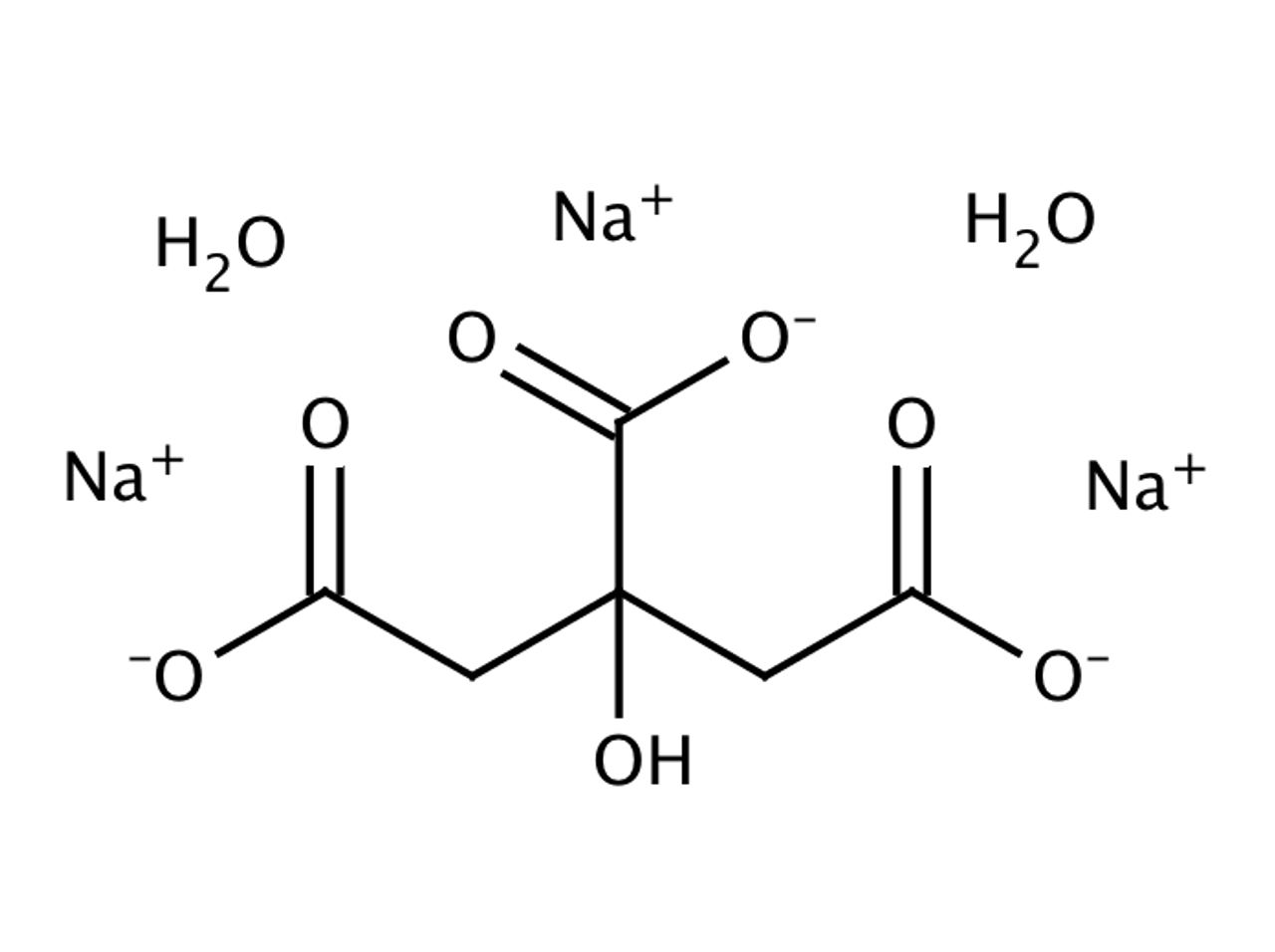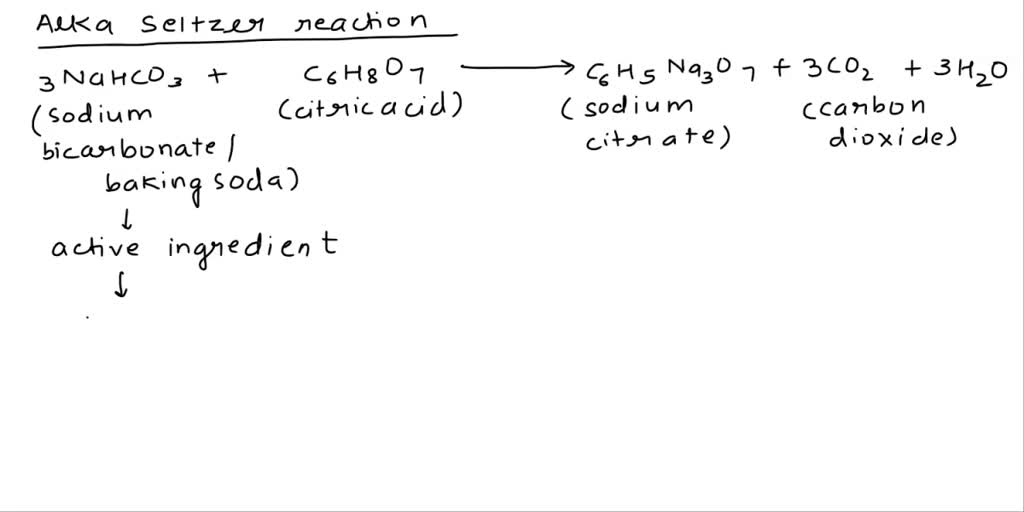
SOLVED: Classify each component of the reaction that occurs when an Alka-Seltzer tablet is dissolving in water: NaHCO3, C6H8O3, H2O, CO2, Na3C6H5O7.
![Phase diagrams for [C4mim]BF4 + Na3C6H5O7 + H2O ABS in the presence of... | Download Scientific Diagram Phase diagrams for [C4mim]BF4 + Na3C6H5O7 + H2O ABS in the presence of... | Download Scientific Diagram](https://www.researchgate.net/publication/325860685/figure/fig4/AS:961857825292304@1606336184782/Phase-diagrams-for-C4mimBF4-Na3C6H5O7-H2O-ABS-in-the-presence-of-different-mass.png)
Phase diagrams for [C4mim]BF4 + Na3C6H5O7 + H2O ABS in the presence of... | Download Scientific Diagram
![Phase diagrams for [C4mim]BF4 + Na3C6H5O7 + H2O ABS in the presence of... | Download Scientific Diagram Phase diagrams for [C4mim]BF4 + Na3C6H5O7 + H2O ABS in the presence of... | Download Scientific Diagram](https://www.researchgate.net/publication/325860685/figure/fig3/AS:961857825275916@1606336184621/Phase-diagrams-for-C4mimBF4-Na3C6H5O7-H2O-ABS-in-the-presence-of-different-mass_Q320.jpg)
Phase diagrams for [C4mim]BF4 + Na3C6H5O7 + H2O ABS in the presence of... | Download Scientific Diagram

SOLVED: The Alka Seltzer reaction involves citric acid and sodium bicarbonate (baking soda) according to the following unbalanced equation: H3C6H5O7 + NaHCO3 -> Na3C6H5O7 + H2O + CO2. Starting from 1.3 g

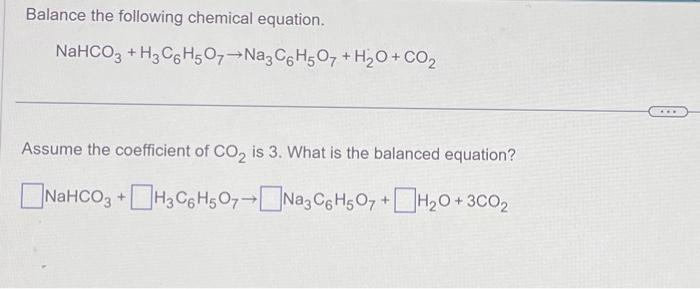
How to Balance H3C6H5O7 + NaHCO3 = CO2 + H2O + Na3C6H5O7 (Citric acid + Sodium bicarbonate ) - YouTube

SOLVED: Soda fizz comes from sodium bicarbonate and citric acid (C6H8O7) reacting to make carbon dioxide, sodium citrate (Na3C6H5O7), and water. Note: the equation is balanced 3 NaHCO3(aq) + C6H8O7(aq) —> 3CO2(g) +
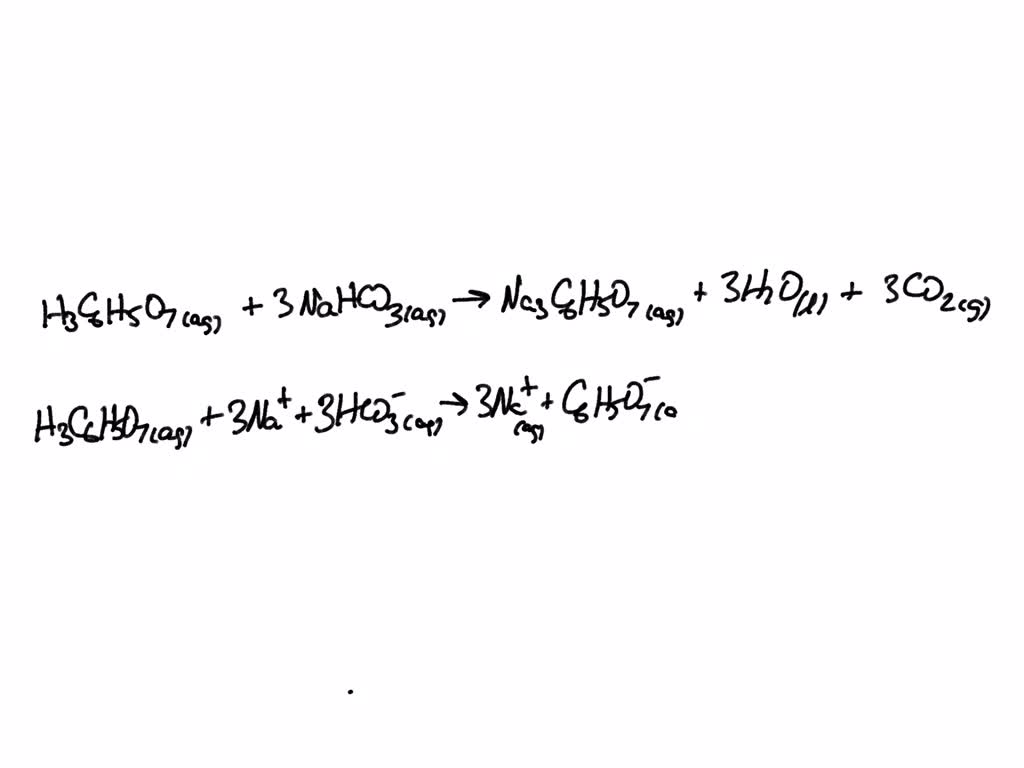
SOLVED: Write the balanced net ionic equation for the reaction between aqueous solutions of citric acid and sodium bicarbonate. H3C6H5O7 (aq) + NaHCO3 (aq) = Na3C6H5O7 (aq) + CO2 (g) + H2O (l)

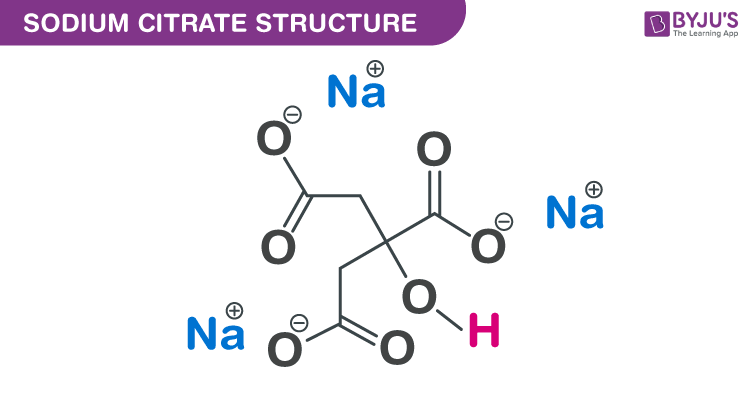
Sodium Citrate Tribasic Dihydrate (Molecular Biology Grade) | CAS 6132-04-3 | SCBT - Santa Cruz Biotechnology

Reduction of HAuCl4 with sodium citrate in water: the sizes of AuNPs... | Download Scientific Diagram

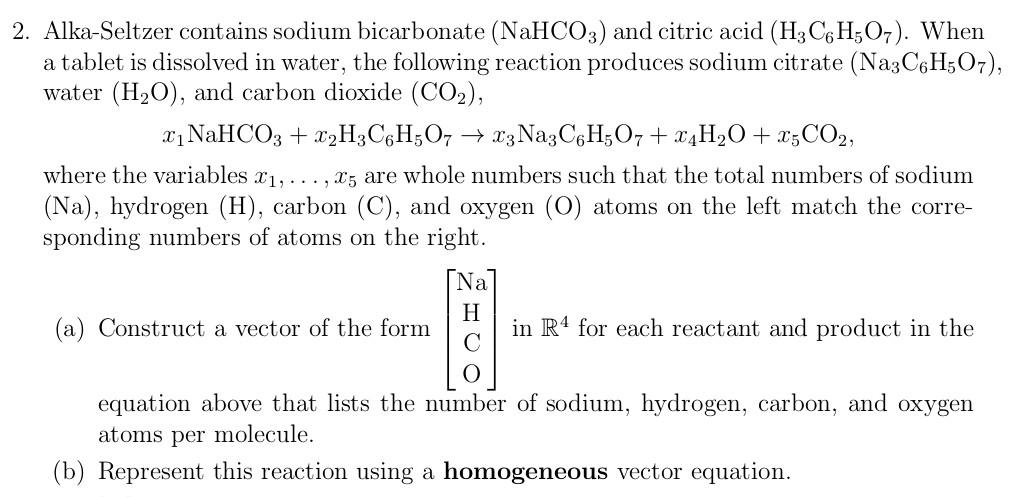
SOLVED: Alka-Seltzer contains sodium bicarbonate (NaHCO3) and citric acid (H3C6H5O7). When the tablet is dissolved in water, the following reaction produces sodium citrate, water, and carbon dioxide (CO2): NaHCO3 + H3C6H5O7 ->