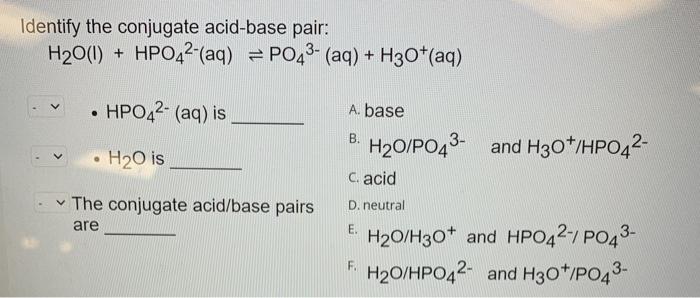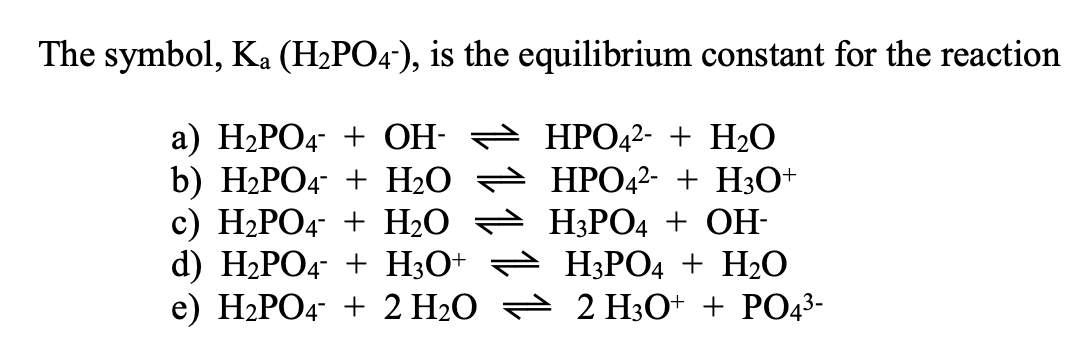![Na2[(VO)2(HPO4)2(C2O4)]·2H2O: A Promising Mixed Polyanionic Cathode Material for Aqueous Zn-Ion Batteries Na2[(VO)2(HPO4)2(C2O4)]·2H2O: A Promising Mixed Polyanionic Cathode Material for Aqueous Zn-Ion Batteries](https://pubs.acs.org/cms/10.1021/acs.inorgchem.2c03308/asset/images/medium/ic2c03308_0004.gif)
Na2[(VO)2(HPO4)2(C2O4)]·2H2O: A Promising Mixed Polyanionic Cathode Material for Aqueous Zn-Ion Batteries
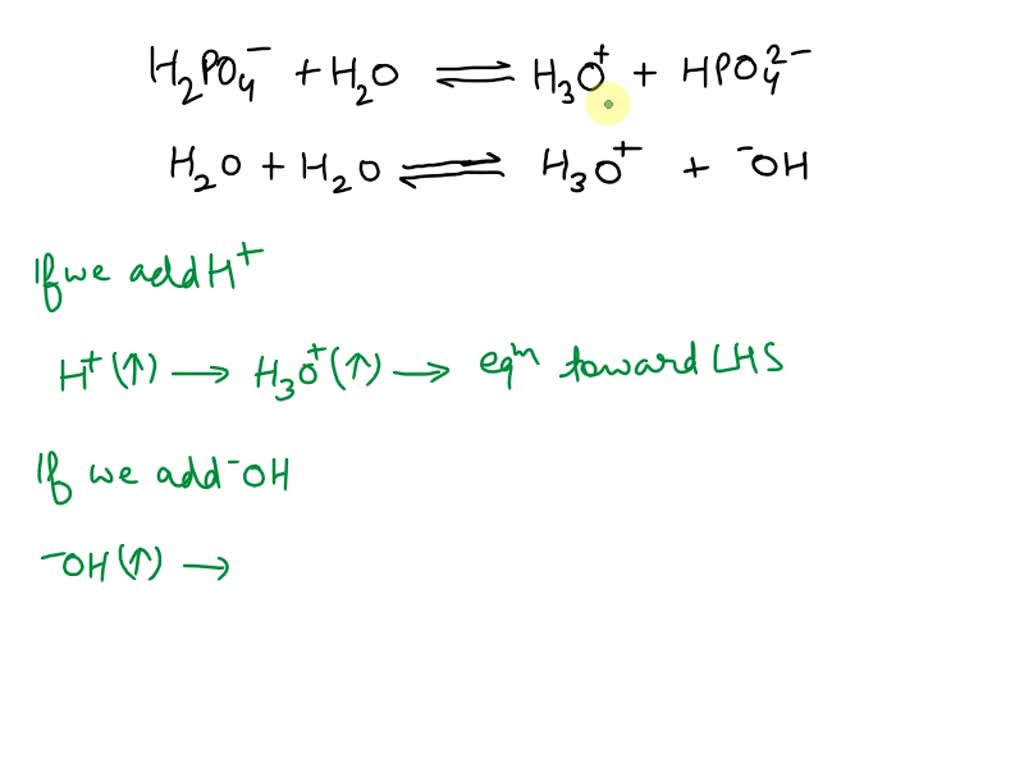
SOLVED: Relevant Equation H2PO4- + H2O <========> H3O+ + HPO4-2 Using Le Chatelier's Principle, explain why the phosphate buffer should have gone through a smaller change pH change compared to distilled water.
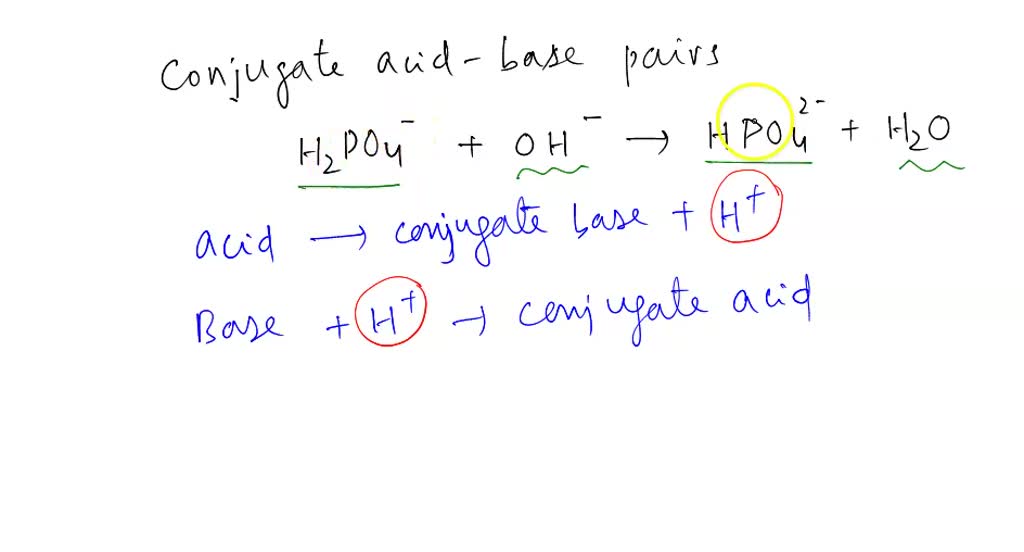
SOLVED: B. Identify the conjugate acid-base pairs in the following reactions: H2PO4- + OH- → HPO4-2 + H2O HBr + H2O → H3O+ + Br- CO3-2 + H2O → HCO3- + OH-
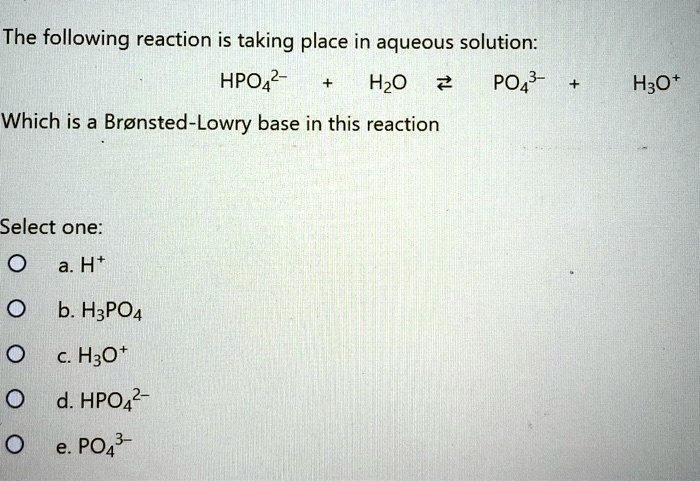
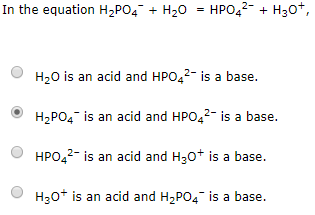
SOLVED: The following reaction is taking place in aqueous solution: HPO42- + H2O -> H3O+ + PO43-. Which is a Bronsted-Lowry base in this reaction? Select one: a. H+ b. H2PO4- c.
CasNo.13772-29-7,Zirconium Phosphate Molecular Formula Zr(HPO4)2·H2O Change The Sewage Become Cleaning Water,(13772-29-7) Suppliers
Synthesis and structural characterisation of solid titanium(IV) phosphate materials by means of X-ray absorption and NMR spectro

Figure 6 from VIIIVIV(HPO4)4·enH·H2O: a mixed-valence vanadium phosphate with an open framework | Semantic Scholar

Nucleation & growth of α-Ti(HPO4)2·H2O single-crystal and its structure determination from X-ray single–crystal data - ScienceDirect

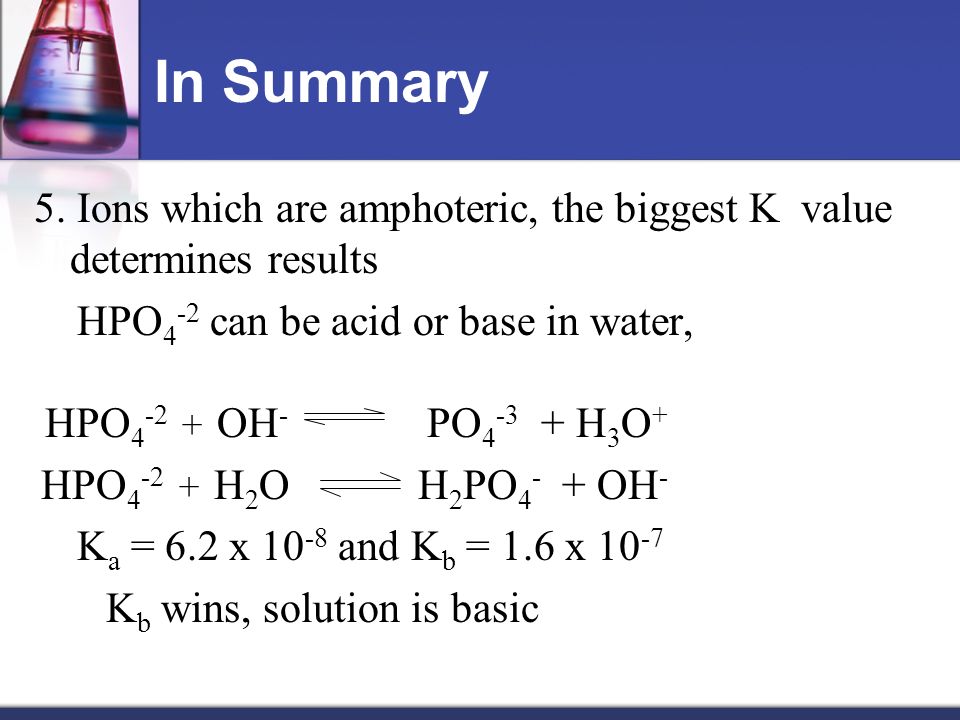
SOLVED: 7. Consider the following equilibrium equations: HPO4 + H2O ↔ H2PO4- + OH- H2PO4- + H2O ↔ HPO4 2- + H3O+ HPO4 2- + H2O ↔ H2PO4- + OH- (1) (2) (

Figure 5. XRD of Zr0.8Ti0.2(HPO4)2.H2O : α- Zirconium Titanium Phosphates - Fibrous Cerium Phosphate Composite Membranes and Their 1,10- Phenanthroline Cu(II) Pillared Materials : Science and Education Publishing

Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol Using Ag@α-Ti(HPO4)2· H2O: Experimental and Computational Studies | Industrial & Engineering Chemistry Research





![Answered: What is the [HPO4-2] of a solution… | bartleby Answered: What is the [HPO4-2] of a solution… | bartleby](https://content.bartleby.com/qna-images/answer/25d6589c-34c4-49fd-a0de-f621c4bd4dbc/9f74c13f-f553-447b-8735-1f1f7effb02f/x03vih.png)