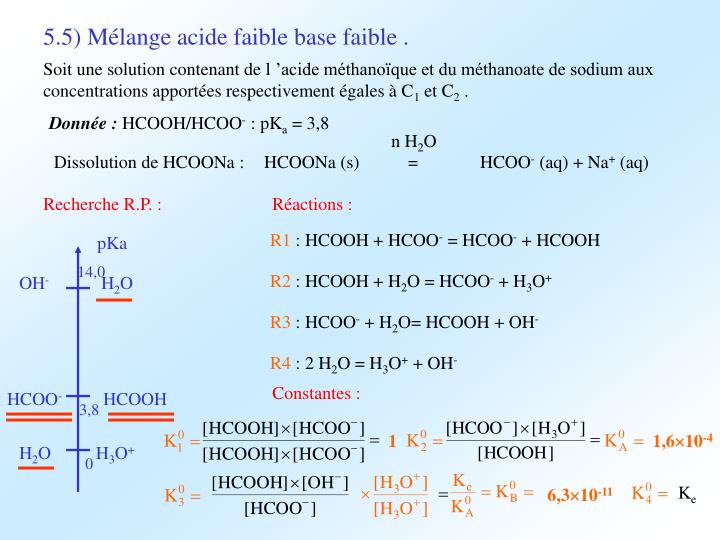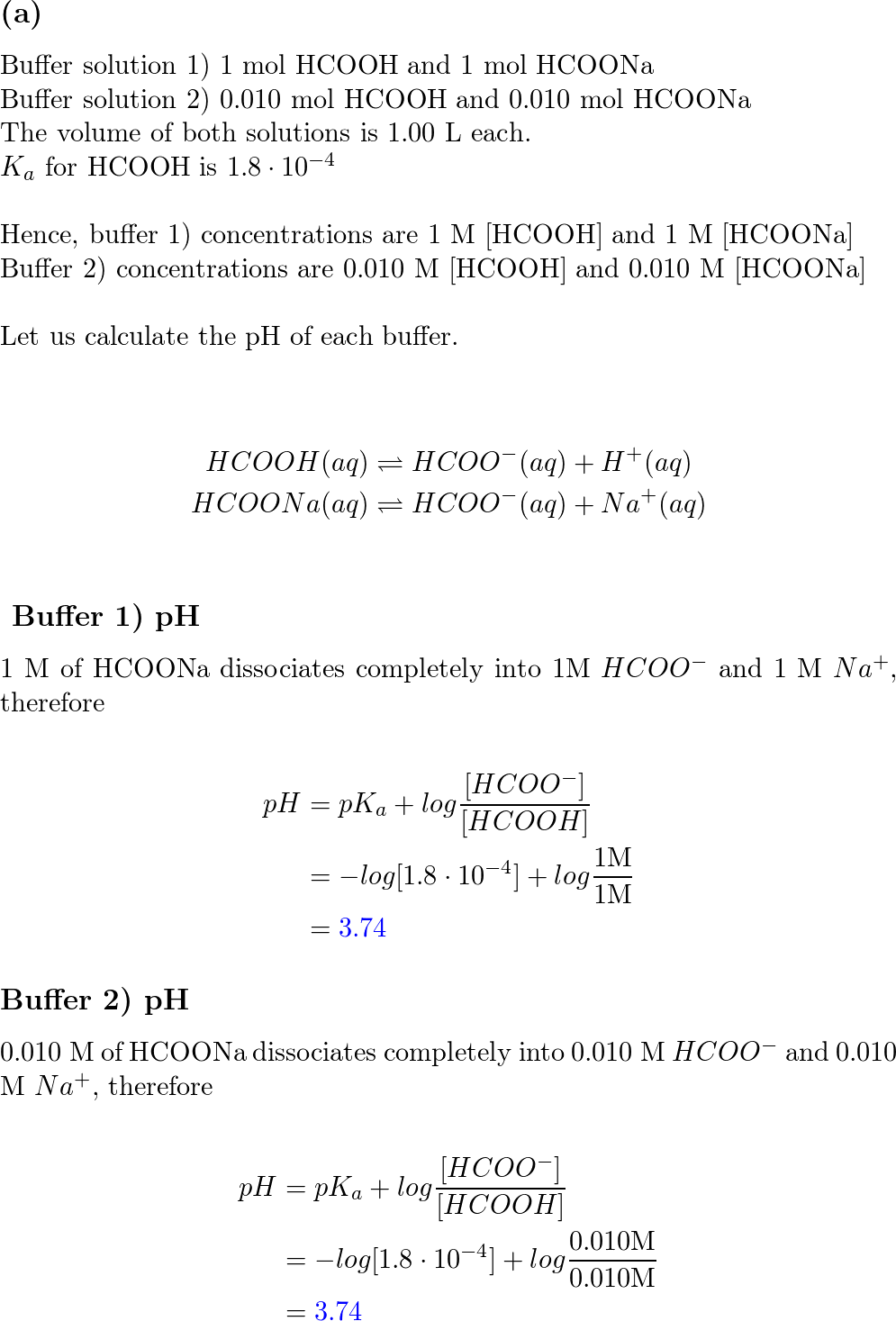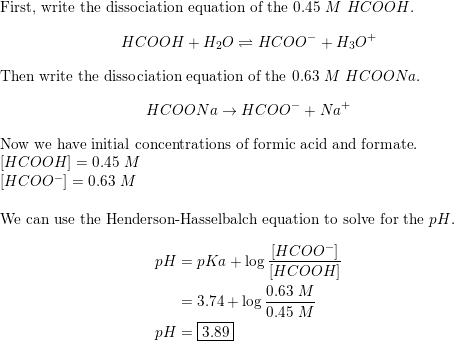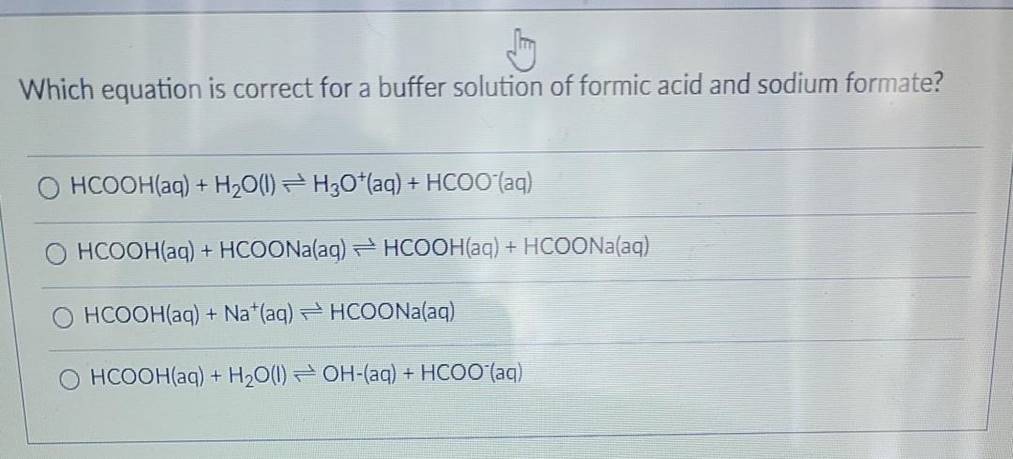
Solved) - Which equation is correct for a buffer solution of formic acid and... (1 Answer) | Transtutors

Experimental phase equilibrium data for the NPG (1) + HCOONa (2) +H2O... | Download Scientific Diagram

Reductive Hydrodehalogenation of Halogenated Carboxylic Acid Derivatives Using a DMSO/HCOONa·2H2O System | Organic Letters
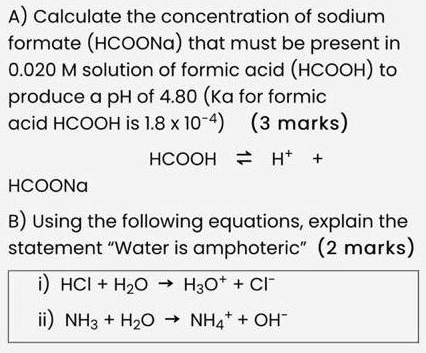
SOLVED: A) Calculate the concentration of sodium formate (HCOONa) that must be present in a 0.020 M solution of formic acid (HCOOH) to produce a pH of 4.80 (Ka for formic acid

Über Das ternäre System HCOOHHCOONaH2O. Zur Kenntnis der sauren Natriumsalze der Ameisensäure - Elöd - 1927 - Zeitschrift für anorganische und allgemeine Chemie - Wiley Online Library

Find the equilibrium constant equilibrium HCOO + H2O HCOOH + OH- In a solution of 0.1 M HCOONa. Ka(HCOOH) = 1.8 * 104 (1) 1.8 x 10-4 (2) 5.56 x 10° (4) 1.8 * 10-18 (3) 5.56 x 107-11

SOLVED: The neutralization of formic acid with aqueous NaOH produces sodium formate (HCOONa) as the only product. The reaction can be represented as follows: HCOOH + NaOH -> HCOONa + H2O.