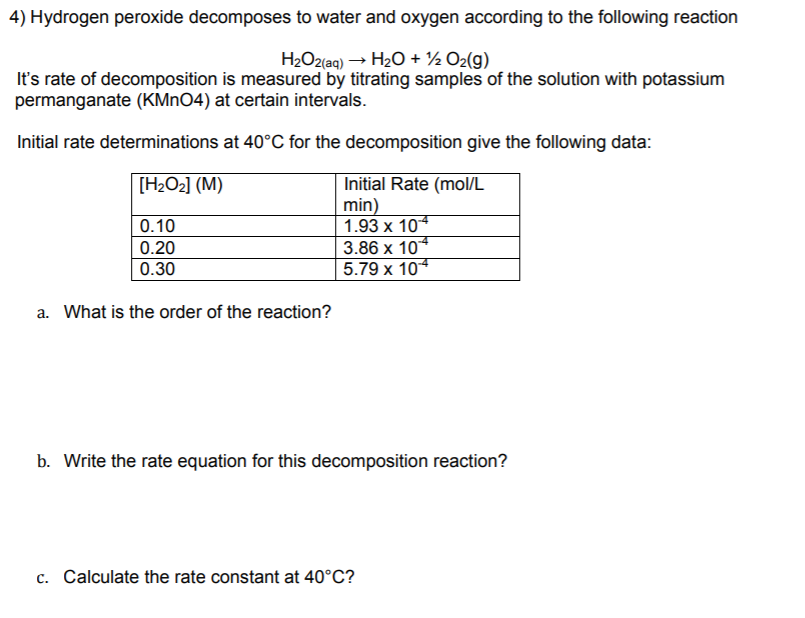
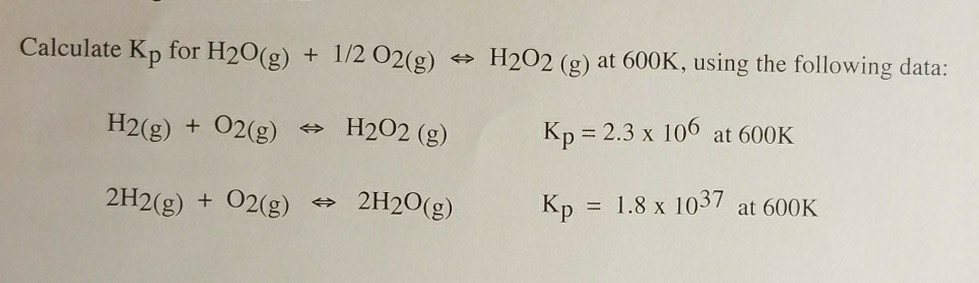I) H2O2 + O3 → H2O + 2O2 (II) H2O2 + Ag2O → 2Ag + H2O + O2 Role of hydrogen peroxide in the - Sarthaks eConnect | Largest Online Education Community
1) H2O2 + O3 → H2O +2O2 2)H2O2 +Ag2O →2Ag +H2O +O2 Determine whether H2O2 is oxidised or reduced in the above reaction? Explain.
✓ Solved: Calculate ΔG^∘ for H2 O(g)+1 / 2 O2(g) ⇌ H2 O2(g) at 600.K using the following data: H2(g)+O2(g)...

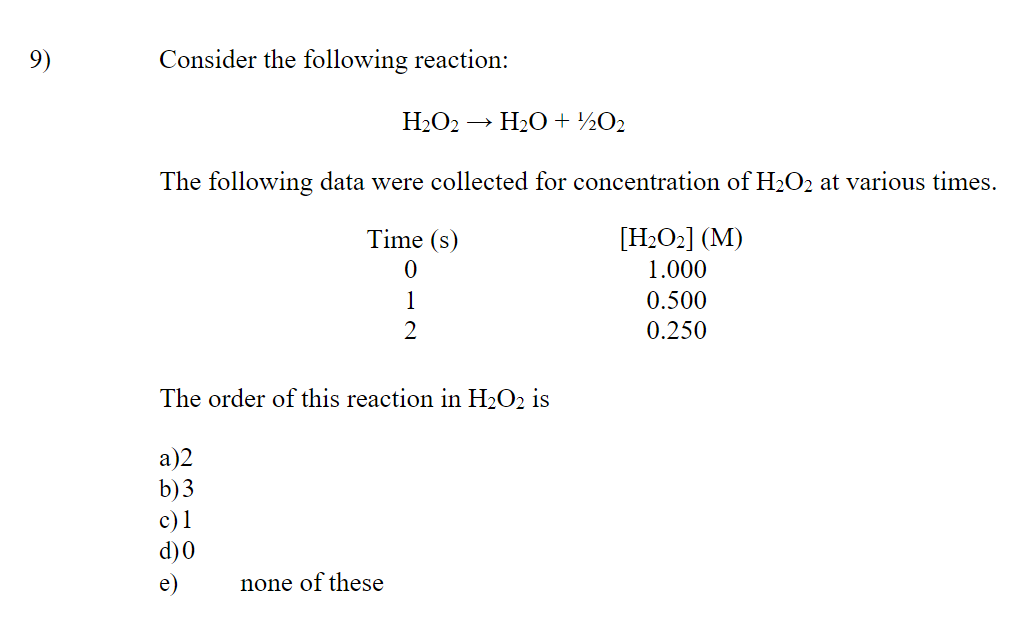
SOLVED: Consider the following chemical reaction mechanism: 1. H2O2 –> H2O + O (slow) 2. O + CF2Cl2. –> ClO + CF2Cl 3. ClO +O3 –> Cl + 2O2 4. Cl +
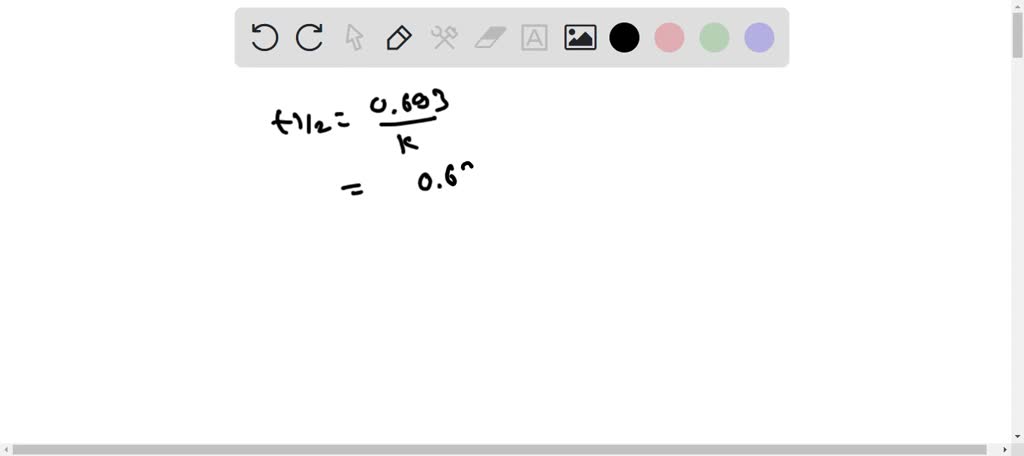
SOLVED: H2O2(aq) ⟶ H2O(l) + 1/2O2(g) a) The above chemical equation is for a first-order reaction. At 303 K, the rate constant equals 9.4 × 10^(-4) s^(-1). Calculate the half-life at this

16.38d | Would hydrogen peroxide be a suitable candidate for fuels: H2O2(l) → H2O(g) + 1/2O2(g) - YouTube

SOLVED: Calculate ΔG° for the reaction H2O(g) + 1/2O2(g) = H2O2(g) at 603 K using the following data: H2(g) + O2(g) = H2O2(g) K = 1.21037 at 603 K 2H2(g) + O2(g) =
Why is the answer B? Can someone explain this to me and why other options are incorrect. I assumed that H2O2 will decompose rapidly to form H20 and O2 with MnO2 as


![Odia] H2O2 to H2O + 1/2 O2 is order reaction. Odia] H2O2 to H2O + 1/2 O2 is order reaction.](https://static.doubtnut.com/ss/web-overlay-thumb/11729098.webp)